Palladium-catalyzed chemoselective allylic substitution, Suzuki-Miyaura cross-coupling, and allene formation of bifunctional 2-B(pin)-substituted allylic acetate derivatives
- PMID: 25077980
- PMCID: PMC4219321
- DOI: 10.1002/chem.201402353
Palladium-catalyzed chemoselective allylic substitution, Suzuki-Miyaura cross-coupling, and allene formation of bifunctional 2-B(pin)-substituted allylic acetate derivatives
Abstract
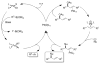
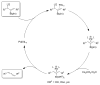
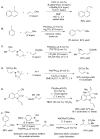
A formidable challenge at the forefront of organic synthesis is the control of chemoselectivity to enable the selective formation of diverse structural motifs from a readily available substrate class. Presented herein is a detailed study of chemoselectivity with palladium-based phosphane catalysts and readily available 2-B(pin)-substituted allylic acetates, benzoates, and carbonates. Depending on the choice of reagents, catalysts, and reaction conditions, 2-B(pin)-substituted allylic acetates and derivatives can be steered into one of three reaction manifolds: allylic substitution, Suzuki-Miyaura cross-coupling, or elimination to form allenes, all with excellent chemoselectivity. Studies on the chemoselectivity of Pd catalysts in their reactivity with boron-bearing allylic acetate derivatives led to the development of diverse and practical reactions with potential utility in synthetic organic chemistry.
Keywords: allenes; allylic substitution; chemoselectivity; cross-coupling; palladium.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
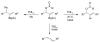
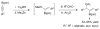
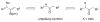
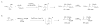
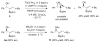
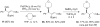

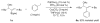
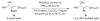
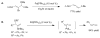
Similar articles
-
Palladium Catalyzed Asymmetric Three-Component Coupling of Boronic Esters, Indoles, and Allylic Acetates.J Am Chem Soc. 2017 May 3;139(17):6038-6041. doi: 10.1021/jacs.7b01410. Epub 2017 Apr 21. J Am Chem Soc. 2017. PMID: 28414430 Free PMC article.
-
Palladium-catalyzed reactions of hypophosphorous compounds with allenes, dienes, and allylic electrophiles: methodology for the synthesis of allylic h-phosphinates.J Org Chem. 2008 Mar 21;73(6):2292-301. doi: 10.1021/jo702542a. Epub 2008 Feb 15. J Org Chem. 2008. PMID: 18275217
-
Pd-catalyzed enantioselective allyl-allyl cross-coupling.J Am Chem Soc. 2010 Aug 11;132(31):10686-8. doi: 10.1021/ja105161f. J Am Chem Soc. 2010. PMID: 20681700 Free PMC article.
-
Palladium-catalyzed cross-coupling reactions of estrone.Steroids. 2025 Jul;219:109627. doi: 10.1016/j.steroids.2025.109627. Epub 2025 May 6. Steroids. 2025. PMID: 40340002 Review.
-
Allylic azides: synthesis, reactivity, and the Winstein rearrangement.Org Biomol Chem. 2019 May 8;17(18):4406-4429. doi: 10.1039/c8ob03178a. Org Biomol Chem. 2019. PMID: 30969292 Free PMC article. Review.
References
-
- Trost BM. Science. 1983;219:245. - PubMed
-
- Wuts PGM, Greene TW. Greene’s Protective Groups in Organic Synthesis. 4. Wiley; Hoboken, NJ: 2007.
-
- Hegedus LS, Söderberg BCG. Transition Metals in the Synthesis of Complex Organic Molecules. University Science Books; Sausalito: 2009.
-
- Trost BM. Angew Chem Int Ed. 1989;28:1173.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

