Osmotic homeostasis
- PMID: 25078421
- PMCID: PMC4422250
- DOI: 10.2215/CJN.10741013
Osmotic homeostasis
Erratum in
-
Correction.Clin J Am Soc Nephrol. 2015 Sep 4;10(9):1703. doi: 10.2215/CJN.08340815. Epub 2015 Aug 19. Clin J Am Soc Nephrol. 2015. PMID: 26289317 Free PMC article. No abstract available.
Abstract
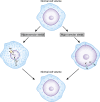
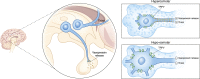
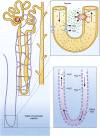
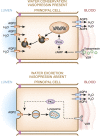
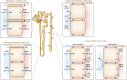
Alterations in water homeostasis can disturb cell size and function. Although most cells can internally regulate cell volume in response to osmolar stress, neurons are particularly at risk given a combination of complex cell function and space restriction within the calvarium. Thus, regulating water balance is fundamental to survival. Through specialized neuronal "osmoreceptors" that sense changes in plasma osmolality, vasopressin release and thirst are titrated in order to achieve water balance. Fine-tuning of water absorption occurs along the collecting duct, and depends on unique structural modifications of renal tubular epithelium that confer a wide range of water permeability. In this article, we review the mechanisms that ensure water homeostasis as well as the fundamentals of disorders of water balance.
Keywords: hypernatremia; hyponatremia; renal physiology; water-electrolyte balance.
Copyright © 2015 by the American Society of Nephrology.
Figures

References
-
- Pitts RF: Physiology of the Kidney and Body Fluids, Chicago, Year Book Medical Publishers, 1963
-
- Silva P, Stoff J, Field M, Fine L, Forrest JN, Epstein FH: Mechanism of active chloride secretion by shark rectal gland: Role of Na-K-ATPase in chloride transport. Am J Physiol 233: F298–F306, 1977 - PubMed
-
- Ortiz RM: Osmoregulation in marine mammals. J Exp Biol 204: 1831–1844, 2001 - PubMed
-
- Crone C, Olesen SP: Electrical resistance of brain microvascular endothelium. Brain Res 241: 49–55, 1982 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

