Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion
- PMID: 25078422
- PMCID: PMC4527031
- DOI: 10.2215/CJN.10311013
Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion
Abstract
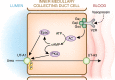
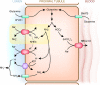
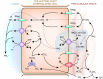
Renal nitrogen metabolism primarily involves urea and ammonia metabolism, and is essential to normal health. Urea is the largest circulating pool of nitrogen, excluding nitrogen in circulating proteins, and its production changes in parallel to the degradation of dietary and endogenous proteins. In addition to serving as a way to excrete nitrogen, urea transport, mediated through specific urea transport proteins, mediates a central role in the urine concentrating mechanism. Renal ammonia excretion, although often considered only in the context of acid-base homeostasis, accounts for approximately 10% of total renal nitrogen excretion under basal conditions, but can increase substantially in a variety of clinical conditions. Because renal ammonia metabolism requires intrarenal ammoniagenesis from glutamine, changes in factors regulating renal ammonia metabolism can have important effects on glutamine in addition to nitrogen balance. This review covers aspects of protein metabolism and the control of the two major molecules involved in renal nitrogen excretion: urea and ammonia. Both urea and ammonia transport can be altered by glucocorticoids and hypokalemia, two conditions that also affect protein metabolism. Clinical conditions associated with altered urine concentrating ability or water homeostasis can result in changes in urea excretion and urea transporters. Clinical conditions associated with altered ammonia excretion can have important effects on nitrogen balance.
Keywords: acidosis; renal physiology; urea.
Copyright © 2015 by the American Society of Nephrology.
Figures





References
-
- Mitch WE, Goldberg AL: Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905, 1996 - PubMed
-
- Lecker SH, Mitch WE: Proteolysis by the ubiquitin-proteasome system and kidney disease. J Am Soc Nephrol 22: 821–824, 2011 - PubMed
-
- Franch HA, Mitch WE: Navigating between the Scylla and Charybdis of prescribing dietary protein for chronic kidney diseases. Annu Rev Nutr 29: 341–364, 2009 - PubMed
-
- Maroni BJ, Steinman TI, Mitch WE: A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 27: 58–65, 1985 - PubMed
-
- Addis T: The ratio between the urea content of the urine and of the blood after the administration of large quantities of urea: An approximate index of the quantity of actively functioning kidney tissue. J Urol 1: 263–287, 1917
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

