Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia
- PMID: 25079173
- PMCID: PMC4360211
- DOI: 10.1038/leu.2014.229
Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia
Abstract
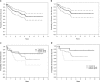
On the basis of the data suggesting that adolescents and young adult patients with acute lymphoblastic leukemia (ALL) have improved outcomes when treated on pediatric protocols, we assessed the feasibility of treating adult patients aged 18-50 years with ALL with the DFCI Pediatric ALL Consortium regimen utilizing a 30-week course of pharmacokinetically dose-adjusted E. coli L-asparaginase during consolidation. Between 2002 and 2008, 92 eligible patients aged 18-50 years were enrolled at 13 participating centers. Seventy-eight patients (85%) achieved a complete remission (CR) after 1 month of intensive induction therapy. With a median follow-up of 4.5 years, the 4-year disease-free survival (DFS) for the patients achieving a CR was 69% (95% confidence interval (CI) 56-78%) and the 4-year overall survival (OS) for all eligible patients was 67% (95% CI 56-76%). The 4-year DFS for the 64 patients who achieved a CR and were Philadelphia chromosome negative (Ph-) was 71% (95% CI 58-81%), and for all 74 Ph- patients the 4-year OS was 70% (95% CI 58-79%). We conclude that a pediatric-like treatment strategy for young adults with de novo ALL is feasible, associated with tolerable toxicity, and results in improved outcomes compared with historical regimens in young adult patients with ALL.
Figures

References
-
- Moricke A, Zimmermann M, Reiter A, Henze G, Schrauder A, Gadner H, et al. Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia. 2010;24:265–284. - PubMed
-
- Vrooman LM, Stevenson KE, Supko JG, O'Brien J, Dahlberg SE, Asselin BL, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study—Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202–1210. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

