Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule
- PMID: 25079250
- PMCID: PMC4237919
- DOI: 10.1186/s13023-014-0124-6
Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by a BKCa channel opener molecule
Abstract
Background: Fragile X Syndrome (FXS) is the most common form of inherited intellectual disability and is also associated with autism spectrum disorders. Previous studies implicated BKCa channels in the neuropathogenesis of FXS, but the main question was whether pharmacological BKCa stimulation would be able to rescue FXS neurobehavioral phenotypes.
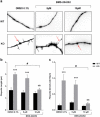
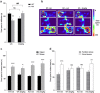
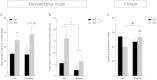
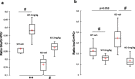
Methods and results: We used a selective BKCa channel opener molecule (BMS-204352) to address this issue in Fmr1 KO mice, modeling the FXS pathophysiology. In vitro, acute BMS-204352 treatment (10 μM) restored the abnormal dendritic spine phenotype. In vivo, a single injection of BMS-204352 (2 mg/kg) rescued the hippocampal glutamate homeostasis and the behavioral phenotype. Indeed, disturbances in social recognition and interaction, non-social anxiety, and spatial memory were corrected by BMS-204352 in Fmr1 KO mice.
Conclusion: These results demonstrate that the BKCa channel is a new therapeutic target for FXS. We show that BMS-204352 rescues a broad spectrum of behavioral impairments (social, emotional and cognitive) in an animal model of FXS. This pharmacological molecule might open new ways for FXS therapy.
Figures
References
-
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. - DOI - PMC - PubMed
-
- Bakker CE, Verheij C, Willemsen R, Helm R, Oerlemans F, Vermey M. Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

