DNA-damage-induced differentiation of leukaemic cells as an anti-cancer barrier
- PMID: 25079327
- PMCID: PMC4410707
- DOI: 10.1038/nature13483
DNA-damage-induced differentiation of leukaemic cells as an anti-cancer barrier
Abstract
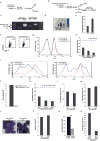
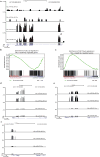
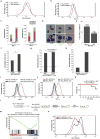
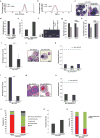
Self-renewal is the hallmark feature both of normal stem cells and cancer stem cells. Since the regenerative capacity of normal haematopoietic stem cells is limited by the accumulation of reactive oxygen species and DNA double-strand breaks, we speculated that DNA damage might also constrain leukaemic self-renewal and malignant haematopoiesis. Here we show that the histone methyl-transferase MLL4, a suppressor of B-cell lymphoma, is required for stem-cell activity and an aggressive form of acute myeloid leukaemia harbouring the MLL-AF9 oncogene. Deletion of MLL4 enhances myelopoiesis and myeloid differentiation of leukaemic blasts, which protects mice from death related to acute myeloid leukaemia. MLL4 exerts its function by regulating transcriptional programs associated with the antioxidant response. Addition of reactive oxygen species scavengers or ectopic expression of FOXO3 protects MLL4(-/-) MLL-AF9 cells from DNA damage and inhibits myeloid maturation. Similar to MLL4 deficiency, loss of ATM or BRCA1 sensitizes transformed cells to differentiation, suggesting that myeloid differentiation is promoted by loss of genome integrity. Indeed, we show that restriction-enzyme-induced double-strand breaks are sufficient to induce differentiation of MLL-AF9 blasts, which requires cyclin-dependent kinase inhibitor p21(Cip1) (Cdkn1a) activity. In summary, we have uncovered an unexpected tumour-promoting role of genome guardians in enforcing the oncogene-induced differentiation blockade in acute myeloid leukaemia.
Figures








Comment in
-
Tumor promoting role of the DNA damage response.Cell Cycle. 2014;13(18):2807-8. doi: 10.4161/15384101.2014.959855. Cell Cycle. 2014. PMID: 25486465 Free PMC article. No abstract available.
Similar articles
-
Bortezomib suppresses self-renewal and leukemogenesis of leukemia stem cell by NF-ĸB-dependent inhibition of CDK6 in MLL-rearranged myeloid leukemia.J Cell Mol Med. 2021 Mar;25(6):3124-3135. doi: 10.1111/jcmm.16377. Epub 2021 Feb 17. J Cell Mol Med. 2021. PMID: 33599085 Free PMC article.
-
IGF signaling contributes to malignant transformation of hematopoietic progenitors by the MLL-AF9 oncoprotein.Exp Hematol. 2012 Sep;40(9):715-723.e6. doi: 10.1016/j.exphem.2012.05.003. Epub 2012 May 18. Exp Hematol. 2012. PMID: 22613471
-
CRISPR Gene Editing of Murine Blood Stem and Progenitor Cells Induces MLL-AF9 Chromosomal Translocation and MLL-AF9 Leukaemogenesis.Int J Mol Sci. 2020 Jun 15;21(12):4266. doi: 10.3390/ijms21124266. Int J Mol Sci. 2020. PMID: 32549410 Free PMC article.
-
MLL translocations, histone modifications and leukaemia stem-cell development.Nat Rev Cancer. 2007 Nov;7(11):823-33. doi: 10.1038/nrc2253. Nat Rev Cancer. 2007. PMID: 17957188 Review.
-
Learning from mouse models of MLL fusion gene-driven acute leukemia.Biochim Biophys Acta Gene Regul Mech. 2020 Aug;1863(8):194550. doi: 10.1016/j.bbagrm.2020.194550. Epub 2020 Apr 19. Biochim Biophys Acta Gene Regul Mech. 2020. PMID: 32320749 Review.
Cited by
-
Noncanonical β-catenin interactions promote leukemia-initiating activity in early T-cell acute lymphoblastic leukemia.Blood. 2023 Mar 30;141(13):1597-1609. doi: 10.1182/blood.2022017079. Blood. 2023. PMID: 36315912 Free PMC article.
-
Stabilization of fatty acid synthesis enzyme acetyl-CoA carboxylase 1 suppresses acute myeloid leukemia development.J Clin Invest. 2021 Jun 15;131(12):e141529. doi: 10.1172/JCI141529. J Clin Invest. 2021. PMID: 34128473 Free PMC article.
-
Wdr5 is essential for fetal erythropoiesis and hematopoiesis.Exp Hematol Oncol. 2023 Apr 15;12(1):39. doi: 10.1186/s40164-023-00385-3. Exp Hematol Oncol. 2023. PMID: 37061728 Free PMC article.
-
DNA damage tolerance in hematopoietic stem and progenitor cells in mice.Proc Natl Acad Sci U S A. 2017 Aug 15;114(33):E6875-E6883. doi: 10.1073/pnas.1706508114. Epub 2017 Jul 31. Proc Natl Acad Sci U S A. 2017. PMID: 28761001 Free PMC article.
-
Targeted sequencing reveals the somatic mutation landscape in a Swedish breast cancer cohort.Sci Rep. 2020 Nov 9;10(1):19304. doi: 10.1038/s41598-020-74580-1. Sci Rep. 2020. PMID: 33168853 Free PMC article.
References
-
- Nijnik A, et al. DNA repair is limiting for haematopoietic stem cells during ageing. Nature. 2007;447:686–690. - PubMed
-
- Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. - PubMed
-
- Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

