Asymmetric synthesis of first generation molecular motors
- PMID: 25079823
- PMCID: PMC5055213
- DOI: 10.1021/ol501925f
Asymmetric synthesis of first generation molecular motors
Abstract
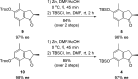
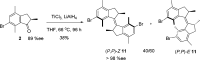
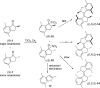
A general enantioselective route to functionalized first generation molecular motors is described. An enantioselective protonation of the silyl enol ethers of indanones by a Au(I)BINAP complex sets the stage for a highly diastereoselective McMurry coupling as a second enhancement step for enantiomeric excess. In this way various functionalized overcrowded alkenes could be synthesized in good yields (up to 78%) and good to excellent enantiomeric excess (85% ee->98% ee) values.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Neilson B. M.; Bielawski C. W. ACS Catal. 2013, 3, 1874–1885.
- Stoll R. S.; Hecht S. Angew. Chem., Int. Ed. 2010, 49, 5054–5075. - PubMed
- Kumagai N.; Shibasaki M. Catal. Sci. Technol. 2013, 3, 41–57.
- Göstl R.; Senf A.; Hecht S. Chem. Soc. Rev. 2014, 43, 1982–1996. - PubMed
- Oudeyer S.; Brière J.-F.; Levacher V.. Eur. J. Org. Chem., doi: 10.1002/ejoc.201402213. - DOI
-
- Harada N.; Koumura N.; Feringa B. L. J. Am. Chem. Soc. 1997, 119, 7256–7264.
- ter Wiel M. K. J.; van Delden R. A.; Meetsma A.; Feringa B. L. J. Am. Chem. Soc. 2003, 125, 15076–15086. - PubMed
-
- McMurry J. E.; Fleming M. P. J. Am. Chem. Soc. 1974, 96, 4708–4709. - PubMed
- Mukaivama T.; Sato T.; Hanna J. Chem. Lett. 1973, 1041–1044.
- Tyrlik S.; Wolochowicz I. Bull. Soc. Chim. Fr. 1973, 2147–2148.
-
Reviews:
- McMurry J. E. Chem. Rev. 1989, 89, 1513–1524.
- Fürstner A.; Bogdanović B. Angew. Chem., Int. Ed. 1996, 35, 2442–2469.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources