Nalbuphine for postoperative pain treatment in children
- PMID: 25079857
- PMCID: PMC10403789
- DOI: 10.1002/14651858.CD009583.pub2
Nalbuphine for postoperative pain treatment in children
Abstract
Background: Several surveys over the past few years have demonstrated that postoperative pain in children is not treated appropriately. One pharmacological treatment option in a multimodal approach for postoperative pain treatment is the systemic administration of opioids. However, opioids are rarely used for postoperative pain treatment in children due to fear of adverse events. One long-standing opioid for systemic use is nalbuphine, a kappa-receptor agonist and µ-receptor antagonist. The efficacy of nalbuphine is believed to be similar to morphine. Increased dosing might result in a ceiling effect, and thus less analgesia than expected. In addition, there might be a lower risk for opioid-induced side effects (nausea, vomiting) and severe adverse events (respiratory depression) due to the antagonistic effect of the µ-receptor. Nalbuphine may be an useful opioid for postoperative use in children, but exact efficacy (e.g. compared to other commonly used opioids) has not been determined yet.
Objectives: To assess the efficacy and adverse events of nalbuphine for acute postoperative pain treatment in children undergoing surgery.
Search methods: We systematically searched the following databases: The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 7), MEDLINE via Pubmed (January 1966 to July 2013) and EMBASE via Ovid (January 1947 to July 2013). We did not impose any restrictions regarding language or publication date. We checked all reference lists of retrieved articles for additional references.
Selection criteria: All randomised controlled trials (RCTs) investigating nalbuphine compared with placebo or other opioids were included.
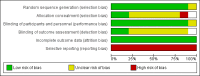
Data collection and analysis: Two review authors independently scanned the retrieved articles and made a decision regarding inclusion or exclusion of studies for this review. The same authors also performed the data extraction and the assessment of risk of bias.
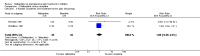
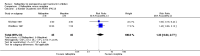
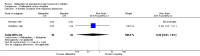
Main results: Ten RCTs including 658 patients were finally included in this systematic review. Five trials compared nalbuphine with placebo. Data from one out of five studies for the outcome moderate/severe pain following nalbuphine compared to placebo gave a risk ratio (RR) 1 hour postoperatively (postop) of 0.1 (95% confidence interval (CI) 0.01 to 0.71; low quality evidence) and a RR 2 hours postop of 0.14 (95% CI 0.02 to 1.06; low quality evidence). The estimated RR based on data from a single study indicated that nalbuphine reduced the requirement for analgesia two hours postop (RR 0.47; 95% CI 0.27 to 0.84; low quality evidence). Two included trials compared nalbuphine with morphine and showed a nonsignificant lower or comparable RR for moderate/severe pain at 1 hour postop (RR 0.84; 95% CI 0.12 to 5.74; low quality evidence), and 2 hours postop (RR 1.09; 95% CI 0.59 to 2.01; low quality evidence) for nalbuphine versus morphine. Four trials compared nalbuphine with tramadol for postoperative pain; data from one trial (per outcome) revealed a lower but nonsignificant RR for the need of additional rescue analgesics in children receiving nalbuphine (RR 2 hours postop 0.75; 95% CI 0.39 to 1.43; low quality evidence) (RR 12 hours postop 0.33; 95% CI 0.04 to 2.77; low quality evidence). One out of three trials comparing nalbuphine with pethidine demonstrated that the RR was not significantly lower following nalbuphine administration compared to pethidine (RR 2 hours postop 1.07; 95% CI 0.52 to 2.23; low quality evidence) (RR 24 hours postop 1.13; 95% CI 0.52 to 2.44; very low quality evidence). The most common adverse event was postoperative nausea and vomiting (PONV). Only one included trial reported that the RR for PONV in the postoperative care unit (PACU) was not significantly higher following nalbuphine compared to placebo (RR 1.00; 95% CI 0.16 to 6.42; low quality evidence) nor to morphine (RR 1.33; 95% CI 0.64 to 2.77; low quality evidence).
Authors' conclusions: Because the overall quality of available evidence was low, this systematic review could not definitively show that the analgesic efficacy of nalbuphine is superior compared to placebo. Furthermore, due to the lack of significant results the comparison with other common opioids is also unclear. The same holds true for the evidence focusing on adverse events following nalbuphine compared to placebo or other opioid administration. The evidence is limited, because studies did not report conclusively all important postoperative pain outcomes (e.g. number of patients with the need for rescue analgesia, postoperative pain scores). Thus, a quantitative analysis was not possible for many major aspects (e.g. rescue analgesia, pain scores) and heterogeneity could not be further explored.
Conflict of interest statement
Alexander Schnabel: No conflicts of interest.
Sylvia Reichl: No conflicts of interest.
Peter Zahn: No conflicts of interest.
Esther Pogatzki‐Zahn: No conflicts of interest.
Figures

































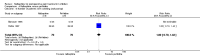
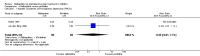



Update of
References
References to studies included in this review
Barsoum 1995 {published data only}
-
- Barsoum MW. Comparison of the efficacy and tolerability of tramadol, pethidine and nalbuphine in children with postoperative pain. Clinical Drug Investigation 1995;9(4):183‐90.
Büttner 1990 {published data only}
-
- Büttner W, Finke W, Schwanitz M, Pfisterer M. Nalbuphine and piritramid in the postoperative phase in young children. 1. General condition. Der Anaesthesist 1990;39(4):211‐6. - PubMed
Galy 1991 {published data only}
-
- Galy A, Rochette A, Beauvoir C, Chardon P, D´Athis F. Intravenous and rectal postoperative nalbuphine analgesia in children. Controlled placebo study. Annals Francaises d'anesthesie et de Reanimation 1991;10 Suppl 1:R104.
Habre 1997 {published data only}
-
- Habre W, McLeod B. Analgesic and respiratory effect of nalbuphine and pethidine for adenotonsillectomy in children with obstructive sleep disorder. Anaesthesia 1997;52(11):1101‐6. - PubMed
Krishnan 1987 {published data only}
-
- Krishnan A, Tolhurst‐Cleaver CL, Kay B. Controlled comparison of nalbuphine and morphine for post‐tonsillectomy pain. Anaesthesia 1985;40(12):1178‐81. - PubMed
Littlejohn 1996 {published data only}
-
- Littlejohn IH, Tarling MM, Flynn PJ, Ordman AJ, Aiken A. Post‐operative pain relief in children following extraction of carious deciduous teeth under general anaesthesia: a comparison of nalbuphine and diclofenac. European Journal of Anaesthesiology 1996;13(4):359‐63. - PubMed
Moyado‐Garcia 2009 {published data only}
-
- Moyao‐García D, Hernández‐Palacios JC, Ramírez‐Mora JC, Nava‐Ocampo AA. A pilot study of nalbuphine versus tramadol administered through continuous intravenous infusion for postoperative pain control in children. Acta Biomedica 2009;80(2):124‐30. - PubMed
Schäffer 1986 {published data only}
-
- Schäffer J, Piepenbrock S, Kretz FJ, Schönfeld C. Nalbuphine and tramadol for the control of postoperative pain in children. Der Anaesthesist 1986;35(7):408‐13. - PubMed
van den Berg 1999 {published data only}
-
- Berg AA, Montoya‐Pelaez LF, Halliday EM, Hassan I, Baloch MS. Analgesia for adenotonsillectomy in children and young adults: a comparison of tramadol, pethidine and nalbuphine. European Journal of Anaesthesiology 1999;16(3):186‐94. - PubMed
Wandless 1987 {published data only}
-
- Wandless JG. A comparison of nalbuphine with morphine for post‐orchidopexy pain. European Journal of Anaesthesiology 1987;4(2):127‐32. - PubMed
References to studies excluded from this review
Bazin 2010 {published data only}
-
- Bazin V, Bollot J, Asehnoune K, Roquilly A, Guillaud C, Windt A, et al. Effects of perioperative intravenous low dose of ketamine on postoperative analgesia in children. European Journal of Anaesthesiology 2010;27:47‐52. - PubMed
Dongmin 2001 {published data only}
-
- Dongmin S, Sukwha K, Chong Sung K, Hee‐Soo K. Postoperative pain management using intravenous patient‐controlled analgesia for pediatric patients. The Journal of craniofacial surgery 2001;12(2):129‐33. - PubMed
Nascimento 2002 {published data only}
-
- Nascimento Junior Pd, Módolo NS, Rodrigues Junior GR. Postoperative analgesia in children less than 1 year of age: a retrospective analysis. Revista Brasileira de Anestesiologia 2002;52:739‐46. - PubMed
Quaki 2007 {published data only}
-
- Ouaki J, Rochette A, Raux O, Dadure Ch, Capdevila X. Tramadol vs nalbuphine: analgesic efficacy and side effects. A prospective, randomized, double‐blinded study in children. European Journal of Anaesthesiology 2007;24:138‐9.
van den Berg 1994 {published data only}
Velegrakis 1998 {published data only}
-
- Velegrakis D, Loizou C, Papageorgiou M, Malissiova A. Comparison of postoperative analgesia in children after continuous i.v. infusion of morphine or nalbuphine through a single‐use infuser (Baxter). British Journal of Anaesthesia 1998;80:A495.
Additional references
Anand 2000
-
- Anand KJ. Pain, plasticity, and premature birth: a prescription for permanent suffering?. Nature Medicine 2000; Vol. 6, issue 9:971‐3. [PUBMED: 10973310] - PubMed
Beaver 1978
-
- Beaver WT, Feise GA. A comparison of the analgesic effect of intramuscular nalbuphine and morphine in patients with postoperative pain. The Journal of Pharmacology and Experimental Therapeutics 1978;204(2):487‐96. [PUBMED: 340643] - PubMed
Bressolle 2011
-
- Bressolle F, Khier S, Rochette A, Kinowski JM, Dadure C, Capdevila X. Population pharmacokinetics of nalbuphine after surgery in children. British Journal of Anaesthesia 2011;106(4):558‐65. [PUBMED: 21310722] - PubMed
Chen 1993
-
- Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sciences 1993;52(4):389‐96. [PUBMED: 8093631] - PubMed
Cummings 1996
-
- Cummings EA, Reid GJ, Finley GA, McGrath PJ, Ritchie JA. Prevalence and source of pain in pediatric inpatients. Pain 1996;68(1):25‐31. [PUBMED: 9251995] - PubMed
Dadure 2009
-
- Dadure C, Bringuier S, Raux O, Rochette A, Troncin R, Canaud N, et al. Continuous peripheral nerve blocks for postoperative analgesia in children: feasibility and side effects in a cohort study of 339 catheters. Canadian Journal of Anaesthesia 2009;56(11):843‐50. [PUBMED: 19697092] - PubMed
Ecoffey 2010
-
- Ecoffey C, Lacroix F, Giaufre E, Orliaguet G, Courreges P, Association des Anesthésistes Réanimateurs Pédiatriques d'Expression Française (ADARPEF). Epidemiology and morbidity of regional anesthesia in children: a follow‐up one‐year prospective survey of the French‐Language Society of Paediatric Anaesthesiologists (ADARPEF). Paediatric Anaesthesia 2010;20(12):1061‐9. [PUBMED: 21199114] - PubMed
Errick 1983
-
- Errick JK, Heel RC. Nalbuphine. A preliminary review of its pharmacological properties and therapeutic efficacy. Drugs 1983;26(3):191‐211. [PUBMED: 6137354] - PubMed
Groenewald 2012
-
- Groenewald CB, Rabbitts JA, Schroeder DR, Harrison TE. Prevalence of moderate‐severe pain in hospitalized children. Paediatric anaesthesia 2012;22(7):661‐8. [PUBMED: 22332912] - PubMed
Higgins 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, March 2011.
Hirschfeld 2013
-
- Hirschfeld G, Zernikow B. Variability of "optimal" cut points for mild, moderate, and severe pain: neglected problems when comparing groups. Pain 2013;154(1):154‐9. [PUBMED: 23182623] - PubMed
Hoskin 1991
-
- Hoskin PJ, Hanks GW. Opioid agonist‐antagonist drugs in acute and chronic pain states. Drugs 1991;41(3):326‐44. [PUBMED: 1711441] - PubMed
Howard 2003
-
- Howard RF. Current status of pain management in children. JAMA 2003;290(18):2464‐9. [PUBMED: 14612483] - PubMed
Jacqz‐Aigrain 2003
-
- Jacqz‐Aigrain E, Debillon T, Daoud P, Boithias C, Hamon I, Rayet I, et al. Population pharmacokinetics of nalbuphine in neonates. Paediatric and Perinatal Drug Therapy 2003;5:190‐8.
Jaillon 1989
-
- Jaillon P, Gardin ME, Lecocq B, Richard MO, Meignan S, Blondel Y, et al. Pharmacokinetics of nalbuphine in infants, young healthy volunteers, and elderly patients. Clinical Pharmacology and Therapeutics 1989;46(2):226‐33. [PUBMED: 2758732] - PubMed
Karling 2002
-
- Karling M, Renstrom M, Ljungman G. Acute and postoperative pain in children: a Swedish nationwide survey. Acta Paediatrica 2002;91(6):660‐6. [PUBMED: 12162598] - PubMed
Kart 1996
-
- Kart T, Laan K, Crombach J, Rasmussen M, Moller KB, Hole P, et al. Postoperative pain management in children has been improved, but can be further optimized. European Journal of Pediatric Surgery: official journal of the Austrian Association of Pediatric Surgery 1996;6(5):259‐64. [PUBMED: 8933127] - PubMed
Michelet 2012
-
- Michelet D, Andreu‐Gallien J, Bensalah T, Hilly J, Wood C, Nivoche Y, et al. A meta‐analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesthesia and analgesia 2012;114(2):393‐406. [PUBMED: 22104069] - PubMed
Miller 1980
-
- Miller RR. Evaluation of nalbuphine hydrochloride. American Journal of Hospital Pharmacy 1980;37(7):942‐9. [PUBMED: 6994499] - PubMed
Rabbitts 2010
-
- Rabbitts JA, Groenewald CB, Moriarty JP, Flick R. Epidemiology of ambulatory anesthesia for children in the United States: 2006 and 1996. Anesthesia and Analgesia 2010;111(4):1011‐5. [PUBMED: 20802051] - PubMed
Review Manager 2012 [Computer program]
-
- The Nordic Cochrane Centre. Review Manager (RevMan) [Computer program]. Version 5.2.. Copenhagen: The Nordic Cochrane Centre, 2012.
Rhodes 1986
-
- Rhodes RA. A cost analysis of nalbuphine versus meperidine used in oral surgery procedures. Clinical Therapeutics 1986;8(4):415‐9. [PUBMED: 3524842] - PubMed
Rushton 1997
-
- Rushton AR, Sneyd JR. Opioid analgesics. British Journal of Hospital Medicine 1997;57(3):105‐6. [PUBMED: 9196589] - PubMed
Schunemann 2008
-
- Schunemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, et al. GRADE: assessing the quality of evidence for diagnostic recommendations. Evidence‐Based Medicine 2008;13(6):162‐3. [PUBMED: 19043023] - PubMed
Segerdahl 2008
-
- Segerdahl M, Warren‐Stomberg M, Rawal N, Brattwall M, Jakobsson J. Children in day surgery: clinical practice and routines. The results from a nation‐wide survey. Acta Anaesthesiologica Scandinavica 2008;52(6):821‐8. [PUBMED: 18498436] - PubMed
Standing 2009
Stringer 2009
-
- Stringer RA, Strain‐Damerell C, Nicklin P, Houston JB. Evaluation of recombinant cytochrome P450 enzymes as an in vitro system for metabolic clearance predictions. Drug Metabolism and Disposition: the Biological Fate of Chemicals 2009;37(5):1025‐34. [PUBMED: 19196847] - PubMed
Taylor 2008
-
- Taylor EM, Boyer K, Campbell FA. Pain in hospitalized children: a prospective cross‐sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Research & Management: the Journal of the Canadian Pain Society 2008;13(1):25‐32. [PUBMED: 18301813] - PMC - PubMed
Walker 2008
-
- Walker SM. Pain in children: recent advances and ongoing challenges. British Journal of Anaesthesia 2008;101(1):101‐10. [PUBMED: 18430745] - PubMed
Weisman 1998
-
- Weisman SJ, Bernstein B, Schechter NL. Consequences of inadequate analgesia during painful procedures in children. Archives of Pediatrics and Adolescent Medicine 1998;152(2):147‐9. [PUBMED: 9491040] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

