Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial
- PMID: 25079928
- PMCID: PMC4120317
- DOI: 10.1136/bmjopen-2014-005094
Cost-effectiveness of ranibizumab and bevacizumab for age-related macular degeneration: 2-year findings from the IVAN randomised trial
Abstract
Objective: To assess the incremental cost and cost-effectiveness of continuous and discontinuous regimens of bevacizumab (Avastin) and ranibizumab (Lucentis) for neovascular age-related macular degeneration (nAMD) from a UK National Health Service (NHS) perspective.
Design: A within-trial cost-utility analysis with a 2-year time horizon, based on a multicentre factorial, non-inferiority randomised controlled trial.
Setting: 23 hospital ophthalmology clinics.
Participants: 610 patients aged ≥50 years with untreated nAMD in the study eye.
Interventions: 0.5 mg ranibizumab or 1.25 mg bevacizumab given continuously (monthly) or discontinuously (as-needed) for 2 years.
Main outcome measures: Quality-adjusted life-years (QALYs).
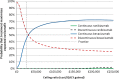
Results: Total 2-year costs ranged from £3002/patient ($4700; 95% CI £2601 to £3403) for discontinuous bevacizumab to £18 590/patient ($29 106; 95% CI £18 258 to £18 922) for continuous ranibizumab. Ranibizumab was significantly more costly than bevacizumab for both continuous (+£14 989/patient ($23 468); 95% CI £14 522 to £15 456; p<0.001) and discontinuous treatment (+£8498 ($13 305); 95% CI £7700 to £9295; p<0.001), with negligible difference in QALYs. Continuous ranibizumab would only be cost-effective compared with continuous bevacizumab if the NHS were willing to pay £3.5 million ($5.5 million) per additional QALY gained. Patients receiving continuous bevacizumab accrued higher total costs (+£599 ($938); 95% CI £91 to £1107; p=0.021) than those receiving discontinuous bevacizumab, but also accrued non-significantly more QALYs (+0.020; 95% CI -0.032 to 0.071; p=0.452). Continuous bevacizumab therefore cost £30 220 ($47 316) per QALY gained versus discontinuous bevacizumab. However, bootstrapping demonstrated that if the NHS is willing to pay £20 000/QALY gained, there is a 37% chance that continuous bevacizumab is cost-effective versus discontinuous bevacizumab.
Conclusions: Ranibizumab is not cost-effective compared with bevacizumab, being substantially more costly and producing little or no QALY gain. Discontinuous bevacizumab is likely to be the most cost-effective of the four treatment strategies evaluated in this UK trial, although there is a 37% chance that continuous bevacizumab is cost-effective.
Trial registration number: ISRCTN92166560.
Keywords: Neovascular age-related macular degeneration (AMD); cost-effectiveness; cost-minimisation analysis; cost-utility analysis; trial-based economic evaluation; vascular endothelial growth factor (VEGF) inhibitors.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


References
-
- Brown DM, Michels M, Kaiser PK, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116:57–65.e5 - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–31 - PubMed
-
- British Medical Association. British National Formulary 62. London: Pharmaceutical Press, 2011. http://bnf.org/bnf/index.htm
-
- Mitchell P. A systematic review of the efficacy and safety outcomes of anti-VEGF agents used for treating neovascular age-related macular degeneration: comparison of ranibizumab and bevacizumab. Curr Med Res Opin 2011;27:1465–75 - PubMed
-
- Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258–67 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
