Equine mesenchymal stromal cells and embryo-derived stem cells are immune privileged in vitro
- PMID: 25080326
- PMCID: PMC4247727
- DOI: 10.1186/scrt479
Equine mesenchymal stromal cells and embryo-derived stem cells are immune privileged in vitro
Abstract
Introduction: Autologous mesenchymal stem cells (MSCs) are an attractive concept in regenerative medicine, but their mechanism of action remains poorly defined. No immune response is reported after in vivo injection of allogeneic equine MSCs or embryo-derived stem cells (ESCs) into the equine tendon, which may be due to the cells' immune-privileged properties. This study further investigates these properties to determine their potential for clinical application in other tissues.
Methods: Mitomycin C-treated MSCs, ESCs, or differentiated ESCs (dESCs) were cultured with allogeneic equine peripheral blood mononuclear cells (PBMCs), and their effect on PBMC proliferation, in the presence or absence of interferon-gamma (IFN-γ) was determined. MSCs and super-antigen (sAg)-stimulated PBMCs were co-cultured directly or indirectly in transwells, and PBMC proliferation examined. Media from MSC culture were harvested and used for PBMC culture; subsequent PBMC proliferation and gene expression were evaluated and media assayed for IFN-γ, tumor necrosis factor alpha (TNF-α), and interleukin (IL)-10 and IL-6 proteins with enzyme-linked immunosorbent assay (ELISA).
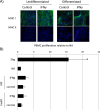
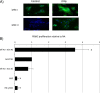
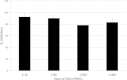
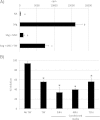
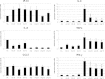
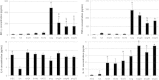
Results: Co-culture of PBMCs with ESCs or dESCs did not affect baseline proliferation, whereas co-culture with MSCs significantly suppressed baseline proliferation. Stimulation of PBMC proliferation by using super-antigens (sAgs) was also suppressed by co-culture with MSCs. Inhibition was greatest with direct contact, but significant inhibition was produced in transwell culture and by using MSC-conditioned media, suggesting that soluble factors play a role in MSC-mediated immune suppression. The MSCs constitutively secrete IL-6, even in the absence of co-culture with PBMCs. MSC-conditioned media also brought about a change in the cytokine-expression profile of sAg-stimulated PBMCs, significantly reducing PBMC expression of IL-6, IFN-γ, and TNF-α.
Conclusions: Equine MSCs and ESCs possess a degree of innate immune privilege, and MSCs secrete soluble factors that suppress PBMC proliferation and alter cytokine expression. These properties may make possible the future clinical use of allogeneic stem cells to help standardize and broaden the scope of treatment of tissue injuries.
Figures

Comment on
-
Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment.Equine Vet J. 2003 Jan;35(1):99-102. doi: 10.2746/042516403775467388. Equine Vet J. 2003. PMID: 12553472 No abstract available.
References
-
- Hankemeier S, Keus M, Zeichen J, Jagodzinski M, Barkhausen T, Bosch U, Krettek C, Van Griensven M. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005;11:41–49. doi: 10.1089/ten.2005.11.41. - DOI - PubMed
-
- Lange-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22:3015–3024. doi: 10.1089/scd.2013.0214. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

