Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation
- PMID: 25080865
- PMCID: PMC4312923
- DOI: 10.1002/ijc.29113
Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation
Abstract
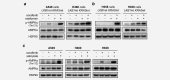
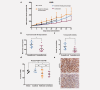
The multikinase inhibitor sorafenib is under clinical investigation for the treatment of many solid tumors, but in most cases, the molecular target responsible for the clinical effect is unknown. Furthermore, enhancing the effectiveness of sorafenib using combination strategies is a major clinical challenge. Here, we identify sorafenib as an activator of AMP-activated protein kinase (AMPK), in a manner that involves either upstream LKB1 or CAMKK2. We further show in a phase II clinical trial in KRAS mutant advanced non-small cell lung cancer (NSCLC) with single agent sorafenib an improved disease control rate in patients using the antidiabetic drug metformin. Consistent with this, sorafenib and metformin act synergistically in inhibiting cellular proliferation in NSCLC in vitro and in vivo. A synergistic effect of both drugs is also seen on phosphorylation of the AMPKα activation site. Our results provide a rationale for the synergistic antiproliferative effects, given that AMPK inhibits downstream mTOR signaling. These data suggest that the combination of sorafenib with AMPK activators could have beneficial effects on tumor regression by AMPK pathway activation. The combination of metformin or other AMPK activators and sorafenib could be tested in prospective clinical trials.
Keywords: AMP-activated protein kinase; metformin; non-small cell lung cancer; salicylate; sorafenib.
© 2014 UICC.
Figures



References
-
- Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

