Increased susceptibility to methotrexate-induced toxicity in nonalcoholic steatohepatitis
- PMID: 25080921
- PMCID: PMC4334808
- DOI: 10.1093/toxsci/kfu156
Increased susceptibility to methotrexate-induced toxicity in nonalcoholic steatohepatitis
Abstract
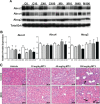
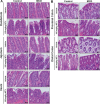
Hepatic drug metabolizing enzymes and transporters play a crucial role in determining the fate of drugs, and alterations in liver function can place individuals at greater risk for adverse drug reactions (ADRs). We have shown that nonalcoholic steatohepatitis (NASH) leads to changes in the expression and localization of enzymes and transporters responsible for the disposition of numerous drugs. The purpose of this study was to determine the effect of NASH on methotrexate (MTX) disposition and the resulting toxicity profile. Sprague Dawley rats were fed either a control or methionine-choline-deficient diet for 8 weeks to induce NASH, then administered a single ip vehicle, 10, 40, or 100 mg/kg MTX injection followed by blood, urine, and feces collection over 96 h with terminal tissue collection. At the onset of dosing, Abcc1-4, Abcb1, and Abcg2 were elevated in NASH livers, whereas Abcc2 and Abcb1 were not properly localized to the membrane, similar to that previously observed in human NASH. NASH rodents receiving 40-100 mg/kg MTX exhibited hepatocellular damage followed by initiation of repair, whereas damage was absent in controls. NASH rodents receiving 100 mg/kg MTX exhibited slightly greater renal toxicity, indicating multiple organ toxicity, despite the majority of the dose being excreted by 6 h. Intestinal toxicity in NASH however, was strikingly less severe than controls, and coincided with reduced fecal MTX excretion. Because MTX-induced gastrointestinal toxicity limits the dose escalation necessary for cancer remission, these data suggest a greater risk for life-threatening MTX-induced hepatic and renal toxicity in NASH in the absence of overt gastrointestinal toxicity.
Keywords: ABC transporters; adverse drug reactions; hepatobiliary disposition; hepatotoxicity; methotrexate.
© The Author 2014. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



References
-
- Anstee Q. M., Daly A. K., Day C. P. Genetic modifiers of non-alcoholic fatty liver disease progression. Biochim. Biophys. Acta. 2011;1812:1557–1566. - PubMed
-
- Barker J., Horn E. J., Lebwohl M., Warren R. B., Nast A., Rosenberg W., Smith C., International Psoriasis, C. Assessment and management of methotrexate hepatotoxicity in psoriasis patients: Report from a consensus conference to evaluate current practice and identify key questions toward optimizing methotrexate use in the clinic. J. Eur. Acad. Dermatol. Venereol. 2011;25:758–764. - PubMed
-
- Butturini A. M., Dorey F. J., Lange B. J., Henry D. W., Gaynon P. S., Fu C., Franklin J., Siegel S. E., Seibel N. L., Rogers P. C., et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J. Clin. Oncol. 2007;25:2063–2069. - PubMed
-
- Chen Z. S., Lee K., Walther S., Raftogianis R. B., Kuwano M., Zeng H., Kruh G. D. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 2002;62:3144–3150. - PubMed
-
- Cheung O., Sanyal A. J. Recent advances in nonalcoholic fatty liver disease. Curr. Opin. Gastroenterol. 2009;25:230–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

