Lung ventilation injures areas with discrete alveolar flooding, in a surface tension-dependent fashion
- PMID: 25080924
- PMCID: PMC4250234
- DOI: 10.1152/japplphysiol.00569.2014
Lung ventilation injures areas with discrete alveolar flooding, in a surface tension-dependent fashion
Abstract
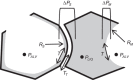
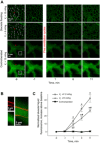
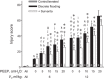
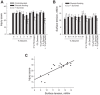
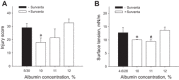
With proteinaceous-liquid flooding of discrete alveoli, a model of the edema pattern in the acute respiratory distress syndrome, lung inflation over expands aerated alveoli adjacent to flooded alveoli. Theoretical considerations suggest that the overexpansion may be proportional to surface tension, T. Yet recent evidence indicates proteinaceous edema liquid may not elevate T. Thus whether the overexpansion is injurious is not known. Here, working in the isolated, perfused rat lung, we quantify fluorescence movement from the vasculature to the alveolar liquid phase as a measure of overdistension injury to the alveolar-capillary barrier. We label the perfusate with fluorescence; micropuncture a surface alveolus and instill a controlled volume of nonfluorescent liquid to obtain a micropunctured-but-aerated region (control group) or a region with discrete alveolar flooding; image the region at a constant transpulmonary pressure of 5 cmH2O; apply five ventilation cycles with a positive end-expiratory pressure of 0-20 cmH2O and tidal volume of 6 or 12 ml/kg; return the lung to a constant transpulmonary pressure of 5 cmH2O; and image for an additional 10 min. In aerated areas, ventilation is not injurious. With discrete alveolar flooding, all ventilation protocols cause sustained injury. Greater positive end-expiratory pressure or tidal volume increases injury. Furthermore, we determine T and find injury increases with T. Inclusion of either plasma proteins or Survanta in the flooding liquid does not alter T or injury. Inclusion of 2.7-10% albumin and 1% Survanta together, however, lowers T and injury. Contrary to expectation, albumin inclusion in our model facilitates exogenous surfactant activity.
Keywords: acute respiratory distress syndrome; plasma proteins; surface tension; surfactant; ventilation injury.
Copyright © 2014 the American Physiological Society.
Figures

References
-
- Bachofen H, Schürch S, Michel RP, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs. Morphol Am Rev Respir Dis 147: 989–996, 1993. - PubMed
-
- Bachofen H, Schürch S, Urbinelli M, Weibel ER. Relations among alveolar surface tension, surface area, volume, and recoil pressure. J Appl Physiol 62: 1878–1887, 1987. - PubMed
-
- Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303: 865–873, 2010. - PubMed
-
- Brower RG, Fessler HE. Another “negative” trial of surfactant. Time to bury this idea? Am J Respir Crit Care Med 183: 966–968, 2011. - PubMed
-
- Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Heart, Lung, and Blood Institute, ARDS Clinical Trials Network. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 351: 327–336, 2004. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

