Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells
- PMID: 25082575
- PMCID: PMC4134946
- DOI: 10.1016/j.jacc.2014.04.056
Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells
Abstract
Background: Human-induced pluripotent stem cells (iPSCs) are a potentially unlimited source for generation of cardiomyocytes (iPSC-CMs). However, current protocols for iPSC-CM derivation face several challenges, including variability in somatic cell sources and inconsistencies in cardiac differentiation efficiency.
Objectives: This study aimed to assess the effect of epigenetic memory on differentiation and function of iPSC-CMs generated from somatic cell sources of cardiac versus noncardiac origins.
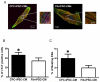
Methods: Cardiac progenitor cells (CPCs) and skin fibroblasts from the same donors were reprogrammed into iPSCs and differentiated into iPSC-CMs via embryoid body and monolayer-based differentiation protocols.
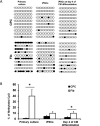
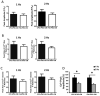
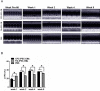
Results: Differentiation efficiency was found to be higher in CPC-derived iPSC-CMs (CPC-iPSC-CMs) than in fibroblast-derived iPSC-CMs (Fib-iPSC-CMs). Gene expression analysis during cardiac differentiation demonstrated up-regulation of cardiac transcription factors in CPC-iPSC-CMs, including NKX2-5, MESP1, ISL1, HAND2, MYOCD, MEF2C, and GATA4. Epigenetic assessment revealed higher methylation in the promoter region of NKX2-5 in Fib-iPSC-CMs compared with CPC-iPSC-CMs. Epigenetic differences were found to dissipate with increased cell passaging, and a battery of in vitro assays revealed no significant differences in their morphological and electrophysiological properties at early passage. Finally, cell delivery into a small animal myocardial infarction model indicated that CPC-iPSC-CMs and Fib-iPSC-CMs possess comparable therapeutic capabilities in improving functional recovery in vivo.
Conclusions: This is the first study to compare differentiation of iPSC-CMs from human CPCs versus human fibroblasts from the same donors. The authors demonstrate that although epigenetic memory improves differentiation efficiency of cardiac versus noncardiac somatic cell sources in vitro, it does not contribute to improved functional outcome in vivo.
Keywords: DNA methylation; cardiac differentiation; epigenetic memory; pluripotent stem cells.
Copyright © 2014 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Epigenetic memory and cardiac cell therapy.J Am Coll Cardiol. 2014 Aug 5;64(5):449-50. doi: 10.1016/j.jacc.2014.05.021. J Am Coll Cardiol. 2014. PMID: 25082576 No abstract available.
References
-
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

