CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models
- PMID: 25082815
- PMCID: PMC4182950
- DOI: 10.1158/0008-5472.CAN-13-3723
CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models
Abstract
Cancer immunotherapy generally offers limited clinical benefit without coordinated strategies to mitigate the immunosuppressive nature of the tumor microenvironment. Critical drivers of immune escape in the tumor microenvironment include tumor-associated macrophages and myeloid-derived suppressor cells, which not only mediate immune suppression, but also promote metastatic dissemination and impart resistance to cytotoxic therapies. Thus, strategies to ablate the effects of these myeloid cell populations may offer great therapeutic potential. In this report, we demonstrate in a mouse model of pancreatic ductal adenocarcinoma (PDAC) that inhibiting signaling by the myeloid growth factor receptor CSF1R can functionally reprogram macrophage responses that enhance antigen presentation and productive antitumor T-cell responses. Investigations of this response revealed that CSF1R blockade also upregulated T-cell checkpoint molecules, including PDL1 and CTLA4, thereby restraining beneficial therapeutic effects. We found that PD1 and CTLA4 antagonists showed limited efficacy as single agents to restrain PDAC growth, but that combining these agents with CSF1R blockade potently elicited tumor regressions, even in larger established tumors. Taken together, our findings provide a rationale to reprogram immunosuppressive myeloid cell populations in the tumor microenvironment under conditions that can significantly empower the therapeutic effects of checkpoint-based immunotherapeutics.
©2014 American Association for Cancer Research.
Figures


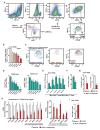

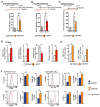


Comment in
-
Macrophage-Targeting by CSF1/1R Blockade in Pancreatic Cancers.Cancer Res. 2021 Dec 15;81(24):6071-6073. doi: 10.1158/0008-5472.CAN-21-3603. Cancer Res. 2021. PMID: 34911778 Free PMC article.
References
-
- Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA113275/CA/NCI NIH HHS/United States
- KL2TR000450/TR/NCATS NIH HHS/United States
- T32 CA 009621/CA/NCI NIH HHS/United States
- T32 CA009621/CA/NCI NIH HHS/United States
- R01 CA177670-01/CA/NCI NIH HHS/United States
- R01 CA168863-01/CA/NCI NIH HHS/United States
- R21 CA182701/CA/NCI NIH HHS/United States
- KL2 TR000450/TR/NCATS NIH HHS/United States
- UL1RR024992/RR/NCRR NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- P30 CA91842/CA/NCI NIH HHS/United States
- R01 CA177670/CA/NCI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- KL2 RR024994/RR/NCRR NIH HHS/United States
- R01 CA168863/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

