Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes
- PMID: 25083336
- PMCID: PMC4108466
- DOI: 10.4161/onci.29244
Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes
Abstract
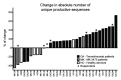
Targeting immune inhibitory receptors has brought excitement, innovation and hope to cancer patients. Our recent work revealed the immunological effects of blocking the CTLA4 and PD-1 immune checkpoints on T cell receptor usage among peripheral blood cells, and further uncovers how the expansion of the T cell repertoire matches the immunotoxicity profile of the therapy.
Keywords: CTLA-4; MK-3475; PBMC; PD-1; TCR; Tremelimumab; sequencing.
Figures
References
-
- Ribas A, Kefford R, Marshall MA, Punt CJ, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–22. doi: 10.1200/JCO.2012.44.6112. - DOI - PMC - PubMed
-
- Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–30. doi: 10.1200/JCO.2013.53.0105. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
