Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models
- PMID: 25083415
- PMCID: PMC4114402
- DOI: 10.1016/S2214-109X(13)70172-4
Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models
Abstract
Background: New WHO guidelines recommend ART initiation for HIV-positive persons with CD4 cell counts ≤500 cells/µL, a higher threshold than was previously recommended. Country decision makers must consider whether to further expand ART eligibility accordingly.
Methods: We used multiple independent mathematical models in four settings-South Africa, Zambia, India, and Vietnam-to evaluate the potential health impact, costs, and cost-effectiveness of different adult ART eligibility criteria under scenarios of current and expanded treatment coverage, with results projected over 20 years. Analyses considered extending eligibility to include individuals with CD4 ≤500 cells/µL or all HIV-positive adults, compared to the previous recommendation of initiation with CD4 ≤350 cells/µL. We assessed costs from a health system perspective, and calculated the incremental cost per DALY averted ($/DALY) to compare competing strategies. Strategies were considered 'very cost-effective' if the $/DALY was less than the country's per capita gross domestic product (GDP; South Africa: $8040, Zambia: $1425, India: $1489, Vietnam: $1407) and 'cost-effective' if $/DALY was less than three times per capita GDP.
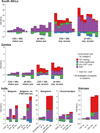
Findings: In South Africa, the cost per DALY averted of extending ART eligibility to CD4 ≤500 cells/µL ranged from $237 to $1691/DALY compared to 2010 guidelines; in Zambia, expanded eligibility ranged from improving health outcomes while reducing costs (i.e. dominating current guidelines) to $749/DALY. Results were similar in scenarios with substantially expanded treatment access and for expanding eligibility to all HIV-positive adults. Expanding treatment coverage in the general population was therefore found to be cost-effective. In India, eligibility for all HIV-positive persons ranged from $131 to $241/DALY and in Vietnam eligibility for CD4 ≤500 cells/µL cost $290/DALY. In concentrated epidemics, expanded access among key populations was also cost-effective.
Interpretation: Earlier ART eligibility is estimated to be very cost-effective in low- and middle-income settings, although these questions should be revisited as further information becomes available. Scaling-up ART should be considered among other high-priority health interventions competing for health budgets.
Funding: The Bill and Melinda Gates Foundation and World Health Organization.
Figures
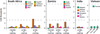


References
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013:269. - PubMed
-
- World Health Organization. Consolidated ARV guidelines 2013: Web annexes. [accessed 4 Aug 2013];2013 http://www.who.int/hiv/pub/guidelines/arv2013/annexes/en/
-
- Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

