Characterization of the hemocytes in Larvae of Protaetia brevitarsis seulensis: involvement of granulocyte-mediated phagocytosis
- PMID: 25083702
- PMCID: PMC4118905
- DOI: 10.1371/journal.pone.0103620
Characterization of the hemocytes in Larvae of Protaetia brevitarsis seulensis: involvement of granulocyte-mediated phagocytosis
Abstract
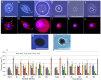
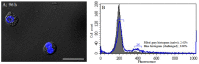
Hemocytes are key players in the immune response against pathogens in insects. However, the hemocyte types and their functions in the white-spotted flower chafers, Protaetia brevitarsis seulensis (Kolbe), are not known. In this study, we used various microscopes, molecular probes, and flow cytometric analyses to characterize the hemocytes in P. brevitarsis seulensis. The circulating hemocytes were classified based on their size, morphology, and dye-staining properties into six types, including granulocytes, plasmatocytes, oenocytoids, spherulocytes, prohemocytes, and adipohemocytes. The percentages of circulating hemocyte types were as follows: 13% granulocytes, 20% plasmatocytes, 1% oenocytoids, 5% spherulocytes, 17% prohemocytes, and 44% adipohemocytes. Next, we identified the professional phagocytes, granulocytes, which mediate encapsulation and phagocytosis of pathogens. The granulocytes were immunologically or morphologically activated and phagocytosed potentially hazardous substances in vivo. In addition, we showed that the phagocytosis by granulocytes is associated with autophagy, and that the activation of autophagy could be an efficient way to eliminate pathogens in this system. We also observed a high accumulation of autophagic vacuoles in activated granulocytes, which altered their shape and led to autophagic cell death. Finally, the granulocytes underwent mitotic division thus maintaining their number in vivo.
Conflict of interest statement
Figures






References
-
- Janeway CA, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20: 197–216. - PubMed
-
- Hoffmann JA (2003) The immune response of Drosophila. Nature 426: 33–38. - PubMed
-
- Tsakas S, Marmaras VJ (2010) Insect immunity and its signaling: an overview. Invertebrate Surv J 7: 228–238.
-
- Silverman N, Maniatis T (2001) NF-κB signaling pathways in mammalian and insect innate immunity. Genes & Dev 15: 2321–2342. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

