Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males
- PMID: 25083989
- PMCID: PMC4151215
- DOI: 10.1172/JCI71048
Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males
Abstract
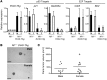
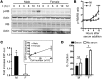
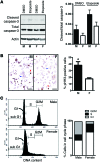
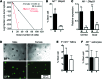
The prevalence of brain tumors in males is common but unexplained. While sex differences in disease are typically mediated through acute sex hormone actions, sex-specific differences in brain tumor rates are comparable at all ages, suggesting that factors other than sex hormones underlie this discrepancy. We found that mesenchymal glioblastoma (Mes-GBM) affects more males as the result of cell-intrinsic sexual dimorphism in astrocyte transformation. We used astrocytes from neurofibromin-deficient (Nf1(-/-)) mice expressing a dominant-negative form of the tumor suppressor p53 (DNp53) and treated them with EGF as a Mes-GBM model. Male Mes-GBM astrocytes exhibited greater growth and colony formation compared with female Mes-GBM astrocytes. Moreover, male Mes-GBM astrocytes underwent greater tumorigenesis in vivo, regardless of recipient mouse sex. Male Mes-GBM astrocytes exhibited greater inactivation of the tumor suppressor RB, higher proliferation rates, and greater induction of a clonogenic, stem-like cell population compared with female Mes-GBM astrocytes. Furthermore, complete inactivation of RB and p53 in Mes-GBM astrocytes resulted in equivalent male and female tumorigenic transformation, indicating that intrinsic differences in RB activation are responsible for the predominance of tumorigenic transformation in male astrocytes. Together, these results indicate that cell-intrinsic sex differences in RB regulation and stem-like cell function may underlie the predominance of GBM in males.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

