p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8⁺ T cells
- PMID: 25083993
- PMCID: PMC4151208
- DOI: 10.1172/JCI75051
p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8⁺ T cells
Abstract
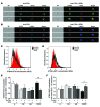
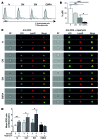
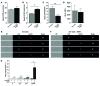
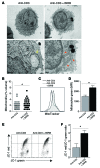
T cell senescence is thought to contribute to immune function decline, but the pathways that mediate senescence in these cells are not clear. Here, we evaluated T cell populations from healthy volunteers and determined that human CD8+ effector memory T cells that reexpress the naive T cell marker CD45RA have many characteristics of cellular senescence, including decreased proliferation, defective mitochondrial function, and elevated levels of both ROS and p38 MAPK. Despite their apparent senescent state, we determined that these cells secreted high levels of both TNF-α and IFN-γ and showed potent cytotoxic activity. We found that the senescent CD45RA-expressing population engaged anaerobic glycolysis to generate energy for effector functions. Furthermore, inhibition of p38 MAPK signaling in senescent CD8+ T cells increased their proliferation, telomerase activity, mitochondrial biogenesis, and fitness; however, the extra energy required for these processes did not arise from increased glucose uptake or oxidative phosphorylation. Instead, p38 MAPK blockade in these senescent cells induced an increase in autophagy through enhanced interactions between p38 interacting protein (p38IP) and autophagy protein 9 (ATG9) in an mTOR-independent manner. Together, our findings describe fundamental metabolic requirements of senescent primary human CD8+ T cells and demonstrate that p38 MAPK blockade reverses senescence via an mTOR-independent pathway.
Figures



References
-
- Liu K, Hodes RJ, Weng N. Cutting edge: telomerase activation in human T lymphocytes does not require increase in telomerase reverse transcriptase (hTERT) protein but is associated with hTERT phosphorylation and nuclear translocation. J Immunol. 2001;166(8):4826–4830. doi: 10.4049/jimmunol.166.8.4826. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

