Golgi UDP-GlcNAc:polypeptide O-α-N-Acetyl-d-glucosaminyltransferase 2 (TcOGNT2) regulates trypomastigote production and function in Trypanosoma cruzi
- PMID: 25084865
- PMCID: PMC4187647
- DOI: 10.1128/EC.00165-14
Golgi UDP-GlcNAc:polypeptide O-α-N-Acetyl-d-glucosaminyltransferase 2 (TcOGNT2) regulates trypomastigote production and function in Trypanosoma cruzi
Abstract
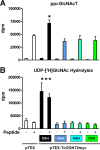
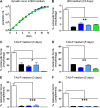
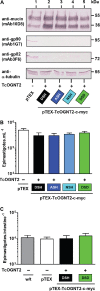
All life cycle stages of the protozoan parasite Trypanosoma cruzi are enveloped by mucin-like glycoproteins which, despite major changes in their polypeptide cores, are extensively and similarly O-glycosylated. O-Glycan biosynthesis is initiated by the addition of αGlcNAc to Thr in a reaction catalyzed by Golgi UDP-GlcNAc:polypeptide O-α-N-acetyl-d-glucosaminyltransferases (ppαGlcNAcTs), which are encoded by TcOGNT1 and TcOGNT2. We now directly show that TcOGNT2 is associated with the Golgi apparatus of the epimastigote stage and is markedly downregulated in both differentiated metacyclic trypomastigotes (MCTs) and cell culture-derived trypomastigotes (TCTs). The significance of downregulation was examined by forced continued expression of TcOGNT2, which resulted in a substantial increase of TcOGNT2 protein levels but only modestly increased ppαGlcNAcT activity in extracts and altered cell surface glycosylation in TCTs. Constitutive TcOGNT2 overexpression had no discernible effect on proliferating epimastigotes but negatively affected production of both types of trypomastigotes. MCTs differentiated from epimastigotes at a low frequency, though they were apparently normal based on morphological and biochemical criteria. However, these MCTs exhibited an impaired ability to produce amastigotes and TCTs in cell culture monolayers, most likely due to a reduced infection frequency. Remarkably, inhibition of MCT production did not depend on TcOGNT2 catalytic activity, whereas TCT production was inhibited only by active TcOGNT2. These findings indicate that TcOGNT2 downregulation is important for proper differentiation of MCTs and functioning of TCTs and that TcOGNT2 regulates these functions by using both catalytic and noncatalytic mechanisms.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Molecular analysis of a UDP-GlcNAc:polypeptide alpha-N-acetylglucosaminyltransferase implicated in the initiation of mucin-type O-glycosylation in Trypanosoma cruzi.Glycobiology. 2009 Aug;19(8):918-33. doi: 10.1093/glycob/cwp068. Epub 2009 May 25. Glycobiology. 2009. PMID: 19468051 Free PMC article.
-
Addition of α-O-GlcNAc to threonine residues define the post-translational modification of mucin-like molecules in Trypanosoma cruzi.Glycoconj J. 2013 Oct;30(7):659-66. doi: 10.1007/s10719-013-9469-7. Epub 2013 Feb 21. Glycoconj J. 2013. PMID: 23430107 Free PMC article. Review.
-
The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms.J Biol Chem. 1995 Nov 10;270(45):27244-53. doi: 10.1074/jbc.270.45.27244. J Biol Chem. 1995. PMID: 7592983
-
Comprehensive glycoprofiling of the epimastigote and trypomastigote stages of Trypanosoma cruzi.J Proteomics. 2017 Jan 16;151:182-192. doi: 10.1016/j.jprot.2016.05.034. Epub 2016 Jun 16. J Proteomics. 2017. PMID: 27318177
-
Trans-sialidase and mucins of Trypanosoma cruzi: an important interplay for the parasite.Carbohydr Res. 2011 Sep 6;346(12):1389-93. doi: 10.1016/j.carres.2011.04.006. Epub 2011 Apr 8. Carbohydr Res. 2011. PMID: 21645882 Review.
Cited by
-
Identification of a Golgi-localized UDP-N-acetylglucosamine transporter in Trypanosoma cruzi.BMC Microbiol. 2015 Nov 21;15:269. doi: 10.1186/s12866-015-0601-7. BMC Microbiol. 2015. PMID: 26589870 Free PMC article.
-
An Iron Transporter Is Involved in Iron Homeostasis, Energy Metabolism, Oxidative Stress, and Metacyclogenesis in Trypanosoma cruzi.Front Cell Infect Microbiol. 2022 Jan 10;11:789401. doi: 10.3389/fcimb.2021.789401. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35083166 Free PMC article.
-
Common and unique features of glycosylation and glycosyltransferases in African trypanosomes.Biochem J. 2022 Sep 16;479(17):1743-1758. doi: 10.1042/BCJ20210778. Biochem J. 2022. PMID: 36066312 Free PMC article.
-
Iron Uptake Controls Trypanosoma cruzi Metabolic Shift and Cell Proliferation.Antioxidants (Basel). 2023 Apr 22;12(5):984. doi: 10.3390/antiox12050984. Antioxidants (Basel). 2023. PMID: 37237850 Free PMC article.
-
Metacyclogenesis as the Starting Point of Chagas Disease.Int J Mol Sci. 2023 Dec 21;25(1):117. doi: 10.3390/ijms25010117. Int J Mol Sci. 2023. PMID: 38203289 Free PMC article. Review.
References
-
- Carvalho-Moreira CJ, Spata MCD, Coura JR, Garcia ES, Azambuja P, Gonzalez MS, Mello CB. 2003. In vivo and in vitro metacyclogenesis tests of two strains of Trypanosoma cruzi in the triatomine vectors Triatoma pseudomaculata and Rhodnius neglectus: short/long-term and comparative study. Exp. Parasitol. 103:102–111. 10.1016/S0014-4894(03)00072-9 - DOI - PubMed
-
- Dias E. 1934. Estudos sobre o Schyzotrypanum cruzi. Mem. Inst. Oswaldo Cruz 28:1–110
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

