A randomized phase II trial of tacrolimus, mycophenolate mofetil and sirolimus after non-myeloablative unrelated donor transplantation
- PMID: 25085357
- PMCID: PMC4181260
- DOI: 10.3324/haematol.2014.108340
A randomized phase II trial of tacrolimus, mycophenolate mofetil and sirolimus after non-myeloablative unrelated donor transplantation
Abstract
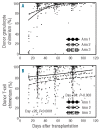
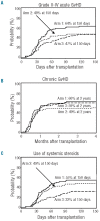
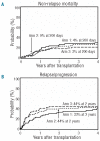
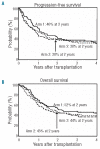
The study is a randomized phase II trial investigating graft-versus-host disease prophylaxis after non-myeloablative (90 mg/m(2) fludarabine and 2 Gy total body irradiation) human leukocyte antigen matched unrelated donor transplantation. Patients were randomized as follows: arm 1 - tacrolimus 180 days and mycophenolate mofetil 95 days (n=69); arm 2 - tacrolimus 150 days and mycophenolate mofetil 180 days (n=71); arm 3 - tacrolimus 150 days, mycophenolate mofetil 180 days and sirolimus 80 days (n=68). All patients had sustained engraftment. Grade II-IV acute graft-versus-host disease rates in the 3 arms were 64%, 48% and 47% at Day 150, respectively (arm 3 vs. arm 1 (hazard ratio 0.62; P=0.04). Owing to the decreased incidence of acute graft-versus-host disease, systemic steroid use was lower at Day 150 in arm 3 (32% vs. 55% in arm 1 and 49% in arm 2; overall P=0.009 by hazard ratio analysis). The Day 150 incidence of cytomegalovirus reactivation was lower in arm 3 (arm 1, 54%; arm 2, 47%; arm 3, 22%; overall P=0.002 by hazard ratio analysis). Non-relapse mortality was comparable in the three arms at two years (arm 1, 26%; arm 2, 23%; arm 3, 18%). Toxicity rates and other outcome measures were similar between the three arms. The addition of sirolimus to tacrolimus and mycophenolate mofetil is safe and associated with lower incidence of acute graft-versus-host disease and cytomegalovirus reactivation. (clinicaltrials.gov identifier: 00105001).
Trial registration: ClinicalTrials.gov NCT00105001.
Copyright© Ferrata Storti Foundation.
Figures

References
-
- Thepot S, Zhou J, Perrot A, Robin M, Xhaard A, de Latour RP, et al. The graft-versus-leukemia effect is mainly restricted to NIH-defined chronic graft-versus-host disease after reduced intensity conditioning before allogeneic stem cell transplantation. Leukemia 2010;24(11):1852–8 - PubMed
-
- Baron F, Maris MB, Sandmaier BM, Storer BE, Sorror M, Diaconescu R, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol 2005;23(9):1993–2003 - PubMed
-
- Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood 1997;89(8):3048–54 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

