Myomaker is essential for muscle regeneration
- PMID: 25085416
- PMCID: PMC4117939
- DOI: 10.1101/gad.247205.114
Myomaker is essential for muscle regeneration
Abstract
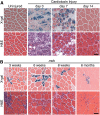
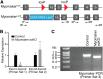
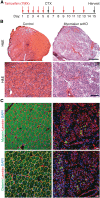
Regeneration of injured adult skeletal muscle involves fusion of activated satellite cells to form new myofibers. Myomaker is a muscle-specific membrane protein required for fusion of embryonic myoblasts, but its potential involvement in adult muscle regeneration has not been explored. We show that myogenic basic helix-loop-helix (bHLH) transcription factors induce myomaker expression in satellite cells during acute and chronic muscle regeneration. Moreover, genetic deletion of myomaker in adult satellite cells completely abolishes muscle regeneration, resulting in severe muscle destruction after injury. Myomaker is the only muscle-specific protein known to be absolutely essential for fusion of embryonic and adult myoblasts.
Keywords: E-box elements; fusion; muscle regeneration; myogenesis; myomaker.
© 2014 Millay et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Berkes CA, Tapscott SJ. 2005. MyoD and the transcriptional control of myogenesis. Semin Cell Dev Biol 16: 585–595 - PubMed
-
- Buckingham M 2006. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev 16: 525–532 - PubMed
-
- Charrin S, Latil M, Soave S, Polesskaya A, Chretien F, Boucheix C, Rubinstein E. 2013. Normal muscle regeneration requires tight control of muscle cell fusion by tetraspanins CD9 and CD81. Nat Commun 4: 1674. - PubMed
-
- Durbeej M, Campbell KP. 2002. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev 12: 349–361 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
