Self-regulation of exopolysaccharide production in Bacillus subtilis by a tyrosine kinase
- PMID: 25085422
- PMCID: PMC4117945
- DOI: 10.1101/gad.246397.114
Self-regulation of exopolysaccharide production in Bacillus subtilis by a tyrosine kinase
Abstract
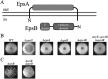
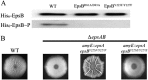
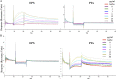
We report that the Bacillus subtilis exopolysaccharide (EPS) is a signaling molecule that controls its own production. EPS synthesis depends on a tyrosine kinase that consists of a membrane component (EpsA) and a kinase component (EpsB). EPS interacts with the extracellular domain of EpsA, which is a receptor, to control kinase activity. In the absence of EPS, the kinase is inactivated by autophosphorylation. The presence of EPS inhibits autophosphorylation and instead promotes the phosphorylation of a glycosyltransferase in the biosynthetic pathway, thereby stimulating the production of EPS. Thus, EPS production is subject to a positive feedback loop that ties its synthesis to its own concentration. Tyrosine kinase-mediated self-regulation could be a widespread feature of the control of exopolysaccharide production in bacteria.
Keywords: biofilm; exopolysaccharide; tyrosine kinase.
© 2014 Elsholz et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
-
- Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. 1990. CD44 is the principal cell surface receptor for hyaluronate. Cell 61: 1303–1313 - PubMed
-
- Bechet E, Guiral S, Torres S, Mijakovic I, Cozzone A-J, Grangeasse C. 2009. Tyrosine-kinases in bacteria: from a matter of controversy to the status of key regulatory enzymes. Amino Acids 37: 499–507 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
