Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes
- PMID: 25085511
- PMCID: PMC4310356
- DOI: 10.1161/JAHA.113.000531
Chronic hypoxemia in late gestation decreases cardiomyocyte number but does not change expression of hypoxia-responsive genes
Abstract
Background: Placental insufficiency is the leading cause of intrauterine growth restriction in the developed world and results in chronic hypoxemia in the fetus. Oxygen is essential for fetal heart development, but a hypoxemic environment in utero can permanently alter development of cardiomyocytes. The present study aimed to investigate the effect of placental restriction and chronic hypoxemia on total number of cardiomyocytes, cardiomyocyte apoptosis, total length of coronary capillaries, and expression of genes regulated by hypoxia.
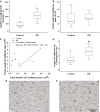
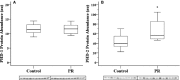
Methods and results: We induced experimental placental restriction from conception, which resulted in fetal growth restriction and chronic hypoxemia. Fetal hearts in the placental restriction group had fewer cardiomyocytes, but interestingly, there was no difference in the percentage of apoptotic cardiomyocytes; the abundance of the transcription factor that mediates hypoxia-induced apoptosis, p53; or expression of apoptotic genes Bax and Bcl2. Likewise, there was no difference in the abundance of autophagy regulator beclin 1 or expression of autophagic genes BECN1, BNIP3, LAMP1, and MAP1LC3B. Furthermore, fetuses exposed to normoxemia (control) or chronic hypoxemia (placental restriction) had similar mRNA expression of a suite of hypoxia-inducible factor target genes, which are essential for angiogenesis (VEGF, Flt1, Ang1, Ang2, and Tie2), vasodilation (iNOS and Adm), and glycolysis (GLUT1 and GLUT3). In addition, there was no change in the expression of PKC-ε, a cardioprotective gene with transcription regulated by hypoxia in a manner independent of hypoxia-inducible factors. There was an increased capillary length density but no difference in the total length of capillaries in the hearts of the chronically hypoxemic fetuses.
Conclusion: The lack of upregulation of hypoxia target genes in response to chronic hypoxemia in the fetal heart in late gestation may be due to a decrease in the number of cardiomyocytes (decreased oxygen demand) and the maintenance of the total length of capillaries. Consequently, these adaptive responses in the fetal heart may maintain a normal oxygen tension within the cardiomyocyte of the chronically hypoxemic fetus in late gestation.
Keywords: angiogenesis; apoptosis; hypoxia; myocytes; pregnancy.
© 2014 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley Blackwell.
Figures


References
-
- Sugishita Y, Leifer DW, Agani F, Watanabe M, Fisher SA. Hypoxia‐responsive signaling regulates the apoptosis‐dependent remodeling of the embryonic avian cardiac outflow tract. Dev Biol. 2004; 273:285-296. - PubMed
-
- Yue X, Tomanek RJ. Stimulation of coronary vasculogenesis/angiogenesis by hypoxia in cultured embryonic hearts. Dev Dyn. 1999; 216:28-36. - PubMed
-
- Semenza GL. HIF‐1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000; 88:1474-1480. - PubMed
-
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008; 30:393-402. - PubMed
-
- An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild‐type p53 by hypoxia‐inducible factor 1alpha. Nature. 1998; 392:405-408. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

