Psoriasis
- PMID: 25085957
- PMCID: PMC4109580
- DOI: 10.1101/cshperspect.a015354
Psoriasis
Abstract
Psoriasis is a common chronic inflammatory skin disease with a spectrum of clinical phenotypes and results from the interplay of genetic, environmental, and immunological factors. Four decades of clinical and basic research on psoriasis have elucidated many of the pathogenic mechanisms underlying disease and paved the way to effective targeted therapies. Here, we review this progress and identify future directions of study that are supported by a more integrative research approach and aim at further improving the patients' life.
Copyright © 2014 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


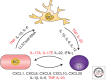
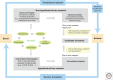
References
-
- Ahlehoff O, Gislason GH, Charlot M, Jorgensen CH, Lindhardsen J, Olesen JB, Abildstrom SZ, Skov L, Torp-Pedersen C, Hansen PR 2011. Psoriasis is associated with clinically significant cardiovascular risk: A Danish nationwide cohort study. J Intern Med 270: 147–157 - PubMed
-
- Amgen. 2013a. Study of efficacy and safety of brodalumab compared with placebo and ustekinumab in moderate to severe plaque psoriasis subjects (AMAGINE-2). clinicaltrials.gov/ct2/show/NCT01708603.
-
- Amgen. 2013b. Study of efficacy and safety of brodalumab compared with placebo and ustekinumab in moderate to severe plaque psoriasis subjects (AMAGINE-3). clinicaltrials.gov/ct2/show/NCT01708629.
-
- Amgen. 2013c. Study of efficacy, safety, and withdrawal and retreatment with brodalumab in moderate to severe plaque psoriasis subjects (AMAGINE-1). http://clinicaltrials.gov/ct2/show/NCT01708590.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
