Pioglitazone normalizes insulin signaling in the diabetic rat retina through reduction in tumor necrosis factor α and suppressor of cytokine signaling 3
- PMID: 25086044
- PMCID: PMC4176235
- DOI: 10.1074/jbc.M114.583880
Pioglitazone normalizes insulin signaling in the diabetic rat retina through reduction in tumor necrosis factor α and suppressor of cytokine signaling 3
Abstract
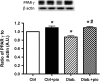
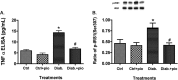
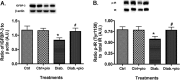
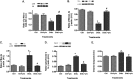
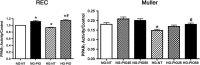
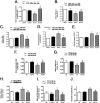
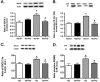
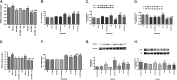
Dysfunctional insulin signaling is a key component of type 2 diabetes. Little is understood of the effects of systemic diabetes on retinal insulin signaling. A number of agents are used to treat patients with type 2 diabetes to normalize glucose levels and improve insulin signaling; however, little has been done to investigate the effects of these agents on retinal insulin signal transduction. We hypothesized that pioglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) agonist, would normalize retinal insulin signal transduction through reduced tumor necrosis factor α (TNFα) and suppressor of cytokine signaling 3 (SOCS3) activities in whole retina and retinal endothelial cells (REC) and Müller cells. To test this hypothesis, we used the BBZDR/Wor type 2 diabetic rat model, as well as REC and Müller cells cultured in normoglycemia and hyperglycemic conditions, to investigate the effects of pioglitazone on TNFα, SOCS3, and downstream insulin signal transduction proteins. We also evaluated pioglitazone's effects on retinal function using electroretinogram and markers of apoptosis. Data demonstrate that 2 months of pioglitazone significantly increased electroretinogram amplitudes in type 2 diabetic obese rats, which was associated with improved insulin receptor activation. These changes occurred in both REC and Müller cells treated with pioglitazone, suggesting that these two cell types are key to insulin resistance in the retina. Taken together, these data provide evidence of impaired insulin signaling in type 2 diabetes rats, which was improved by increasing PPARγ activity. Further investigations of PPARγ actions in the retina may provide improved treatment options.
Keywords: Diabetic Retinopathy; Insulin Receptor; Insulin Receptor Substrate 1 (IRS-1); Insulin Resistance; Suppressor of Cytokine Signaling 3 (SOCS3); Tumor Necrosis Factor (TNF); Type 2 Diabetes.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C., Mullany E. C., Biryukov S., Abbafati C., Abera S. F., Abraham J. P., Abu-Rmeileh N. M., Achoki T., AlBuhairan F. S., Alemu Z. A., Alfonso R., Ali M. K., Ali R., Guzman N. A., Ammar W., Anwari P., Banerjee A., Barquera S., Basu S., Bennett D. A., Bhutta Z., Blore J., Cabral N., Nonato I. C., Chang J. C., Chowdhury R., Courville K. J., Criqui M. H., Cundiff D. K., Dabhadkar K. C., Dandona L., Davis A., Dayama A., Dharmaratne S. D., Ding E. L., Durrani A. M., Esteghamati A., Farzadfar F., Fay D. F., Feigin V. L., Flaxman A., Forouzanfar M. H., Goto A., Green M. A., Gupta R., Hafezi-Nejad N., Hankey G. J., Harewood H. C., Havmoeller R., Hay S., Hernandez L., Husseini A., Idrisov B. T., Ikeda N., Islami F., Jahangir E., Jassal S. K., Jee S. H., Jeffreys M., Jonas J. B., Kabagambe E. K., Khalifa S. E., Kengne A. P., Khader Y. S., Khang Y. H., Kim D., Kimokoti R. W., Kinge J. M., Kokubo Y., Kosen S., Kwan G., Lai T., Leinsalu M., Li Y., Liang X., Liu S., Logroscino G., Lotufo P. A., Lu Y., Ma J., Mainoo N. K., Mensah G. A., Merriman T. R., Mokdad A. H., Moschandreas J., Naghavi M., Naheed A., Nand D., Narayan K. M., Nelson E. L., Neuhouser M. L., Nisar M. I., Ohkubo T., Oti S. O., Pedroza A., Prabhakaran D., Roy N., Sampson U., Seo H., Sepanlou S. G., Shibuya K., Shiri R., Shiue I., Singh G. M., Singh J. A., Skirbekk V., Stapelberg N. J., Sturua L., Sykes B. L., Tobias M., Tran B. X., Trasande L., Toyoshima H., van de Vijver S., Vasankari T. J., Veerman J. L., Velasquez-Melendez G., Vlassov V. V., Vollset S. E., Vos T., Wang C., Wang S. X., Weiderpass E., Werdecker A., Wright J. L., Yang Y. C., Yatsuya H., Yoon J., Yoon S. J., Zhao Y., Zhou M., Zhu S., Lopez A. D., Murray C. J., Gakidou E. (2014) Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 10.1016/S0140-6736(14)60460-8 - DOI - PMC - PubMed
-
- Tawfik A., Sanders T., Kahook K., Akeel S., Elmarakby A., Al-Shabrawey M. (2009) Suppression of retinal peroxisome proliferator-activated receptor γ in experimental diabetes and oxygen-induced retinopathy: role of NADPH oxidase. Invest. Ophthalmol. Vis. Sci. 50, 878–884 - PubMed
-
- Yau H., Rivera K., Lomonaco R., Cusi K. (2013) The future of thiazolidinedione therapy in the management of type 2 diabetes mellitus. Curr. Diab. Rep. 13, 329–341 - PubMed
-
- Iwata M., Haruta T., Usui I., Takata Y., Takano A., Uno T., Kawahara J., Ueno E., Sasaoka T., Ishibashi O., Kobayashi M. (2001) Pioglitazone ameliorates tumor necrosis factor-α-induced insulin resistance by a mechanism independent of adipogenic activity of peroxisome proliferator-activated receptor-γ. Diabetes 50, 1083–1092 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

