Ultrashort pulsed laser treatment inactivates viruses by inhibiting viral replication and transcription in the host nucleus
- PMID: 25086212
- PMCID: PMC4171215
- DOI: 10.1016/j.antiviral.2014.07.012
Ultrashort pulsed laser treatment inactivates viruses by inhibiting viral replication and transcription in the host nucleus
Abstract
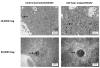
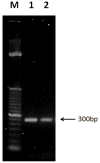
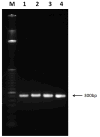
Ultrashort pulsed laser irradiation is a new method for virus reduction in pharmaceuticals and blood products. Current evidence suggests that ultrashort pulsed laser irradiation inactivates viruses through an impulsive stimulated Raman scattering process, resulting in aggregation of viral capsid proteins. However, the specific functional defect(s) in viruses inactivated in this manner have not been demonstrated. This information is critical for the optimization and the extension of this treatment platform to other applications. Toward this goal, we investigated whether viral internalization, replication, or gene expression in cells were altered by ultrashort pulsed laser irradiation. Murine Cytomegalovirus (MCMV), an enveloped DNA virus, was used as a model virus. Using electron and fluorescence microscopy, we found that laser-treated MCMV virions successfully internalized in cells, as evidenced by the detection of intracellular virions, which was confirmed by the detection of intracellular viral DNA via PCR. Although the viral DNA itself remained polymerase-amplifiable after laser treatment, no viral replication or gene expression was observed in cells infected with laser-treated virus. These results, along with evidence from previous studies, support a model whereby the laser treatment stabilizes the capsid, which inhibits capsid uncoating within cells. By targeting the mechanical properties of viral capsids, ultrashort pulsed laser treatment represents a unique potential strategy to overcome viral mutational escape, with implications for combatting emerging or drug-resistant pathogens.
Keywords: Murine cytomegalovirus; Pathogen inactivation; Pathogen reduction; Ultrashort pulsed lasers.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
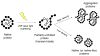




References
-
- Tsen K, Tsen SWD, Hung CF, Wu T, Kiang JG. Selective inactivation of human immunodeficiency virus with subpicosecond near-infrared laser pulses. Journal of Physics: Condensed Matter. 2008;20:252205.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

