Initial study of radiological and clinical efficacy radioembolization using 188Re-human serum albumin (HSA) microspheres in patients with progressive, unresectable primary or secondary liver cancers
- PMID: 25086245
- PMCID: PMC4136939
- DOI: 10.12659/MSM.890480
Initial study of radiological and clinical efficacy radioembolization using 188Re-human serum albumin (HSA) microspheres in patients with progressive, unresectable primary or secondary liver cancers
Abstract
Background: The aim of this initial study was to evaluate the clinical and radiological effectiveness of radioembolization (RE) using 188Re-Human Serum Albumin (HSA) microspheres in patients with advanced, progressive, unresectable primary or secondary liver cancers, not suitable to any other form of therapy.
Material/methods: Overall, we included 13 patients with 20 therapy sessions. Clinical and radiological responses were assessed at 6 weeks after therapy, and then every 3 months. The objective radiological response was classified according to Response Evaluation Criteria in Solid Tumors (RECIST) v.1.0 by sequential MRI. Adverse events were evaluated using NCI CTCAE v.4.03.
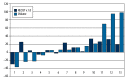
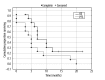
Results: There were 4 patients with hepatocellular carcinoma (HCC), 6 with metastatic colorectal cancer (mCRC), 2 with neuroendocrine carcinoma (NEC), and 1 patient with ovarian carcinoma. Mean administered activity of 188Re HSA was 7.24 GBq (range 3.8-12.4) A high microspheres labeling efficacy of over 97±2.1% and low urinary excretion of 188Re (6.5±2.3%) during first 48-h follow-up. Median overall survival (OS) for all patients was 7.1 months (CI 6.2-13.3) and progression-free survival (PFS) was 5.1 months (CI 2.4-9.9). In those patients who had a clinical partial response (PR), stable disease (SD), and disease progression (DP) as assessed 6 weeks after therapy, the median OS was 9/5/4 months, respectively, and PFS was 5/2/0 months, respectively. The treatment adverse events (toxicity) were at an acceptable level. Initially and after 6 weeks, the CTC AE was grade 2, while after 3 months it increased to grade 3 in 4 subjects. This effect was mostly related to rapid cancer progression in this patient subgroup.
Conclusions: The results of this preliminary study indicate that RE using 188Re HSA is feasible and a viable option for palliative therapy in patients with extensive progressive liver cancer. It was well tolerated by most patients, with a low level of toxicity during the 3 months of follow-up.
Figures


References
-
- Kennedy AS, Coldwell D, Nutting C, et al. Resin Y-90-microsphere brachytherapy for unresectable colorectal liver metastases: Modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412–25. - PubMed
-
- Stubbs RS, Cannan RJ, Mitchell AW. Selective internal radiation therapy (SIRT) with 90Yttrium microspheres for extensive colorectal liver metastases. Hepatogastroenterology. 2001;48:333–37. - PubMed
-
- Salem R, Thurston KG, Carr BI, et al. Yttrium-90 microspheres: Radiation therapy for unresectable liver cancer. J Vasc Interv Radio. 2002;13:S223–29. - PubMed
-
- Shcherbinin S, Celler A, Belhocine T, et al. Accuracy of Quantitative Reconstructions in SPECT/CT Imaging. Phys Med Biol. 2008;53:4595–604. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

