Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients
- PMID: 25086573
- PMCID: PMC11022119
- DOI: 10.1002/14651858.CD009036.pub2
Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients
Abstract
Background: Genital herpes is caused by herpes simplex virus 1 (HSV-1) or 2 (HSV-2). Some infected people experience outbreaks of genital herpes, typically, characterized by vesicular and erosive localized painful genital lesions.
Objectives: To compare the effectiveness and safety of three oral antiviral drugs (acyclovir, famciclovir and valacyclovir) prescribed to suppress genital herpes outbreaks in non-pregnant patients.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the search portal of the World Health Organization International Clinical Trials Registry Platform and pharmaceutical company databases up to February 2014. We also searched US Food and Drug Administration databases and proceedings of seven congresses to a maximum of 10 years. We contacted trial authors and pharmaceutical companies.
Selection criteria: We selected parallel-group and cross-over randomized controlled trials including patients with recurrent genital herpes caused by HSV, whatever the type (HSV-1, HSV-2, or undetermined), with at least four recurrences per year (trials concerning human immunodeficiency virus (HIV)-positive patients or pregnant women were not eligible) and comparing suppressive oral antiviral treatment with oral acyclovir, famciclovir, and valacyclovir versus placebo or another suppressive oral antiviral treatment.
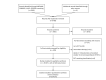
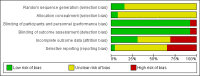
Data collection and analysis: Two review authors independently selected eligible trials and extracted data. The Risk of bias tool was used to assess risk of bias. Treatment effect was measured by the risk ratio (RR) of having at least one genital herpes recurrence. Pooled RRs were derived by conventional pairwise meta-analyses. A network meta-analysis allowed for estimation of all possible two-by-two comparisons between antiviral drugs.
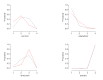
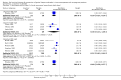
Main results: A total of 26 trials (among which six had a cross-over design) were included. Among the 6950 randomly assigned participants, 54% (range 0 to 100%) were female, mean age was 35 years (range 26 to 45.1), and the mean number of recurrences per year was 11 (range 6.3 to 17.8). Duration of treatment was two to 12 months. Risk of bias was considered high for half of the studies and unclear for the other half. A total of 14 trials compared acyclovir versus placebo, four trials compared valacyclovir versus placebo and 2 trials compared valacyclovir versus no treatment. Three trials compared famciclovir versus placebo. Two trials compared valacyclovir versus famciclovir and one trial compared acyclovir versus valacyclovir versus placebo.We analyzed data from 22 trials for the outcome: risk of having at least one clinical recurrence. We could not obtain the outcome data for four trials. In placebo-controlled trials, there was a low quality evidence that the risk of having at least one clinical recurrence was reduced with acyclovir (nine parallel-group trials, n = 2049; pooled RR 0.48, 95% confidence interval (CI) 0.39 to 0.58), valacyclovir (four trials, n = 1788; pooled RR 0.41, 95% CI 0.24 to 0.69), or famciclovir (two trials, n = 732; pooled RR 0.57, 95% CI 0.50 to 0.64). The six cross-over trials showed larger treatment effects on average than the parallel-group trials. We found evidence of a small-study effect for acyclovir placebo-controlled trials (adjusted pooled RR 0.61, 95% CI 0.49 to 0.75). In analyzing parallel-group trials by daily dose, no clear evidence was found of a dose-response relationship for any drug. In head-to-head trials, the risk of having at least one recurrence was increased with valacyclovir rather than acyclovir (one trial, n = 1345; RR 1.16, 95% CI 1.01 to 1.34) and was not significantly different from that seen with famciclovir as compared with valacyclovir (one trial, n = 320; RR 1.18, 95% CI 0.86 to 1.63).We included 16 parallel-arm trials in a network meta-analysis and we were unable to determine which of the drugs was most effective in reducing the risk of at least one clinical recurrence (after adjustment for small-study effects, pooled RR 0.83, 95% CI 0.61 to 1.11 for valacyclovir vs acyclovir; pooled RR 1.04, 95% CI, 0.71 to 1.49 for famciclovir vs acyclovir; and pooled RR 1.26, 95% CI 0.89 to 1.75 for famciclovir vs valacyclovir). Safety data were sought but were reported as total numbers of adverse events.
Authors' conclusions: Owing to risk of bias and inconsistency, there is low quality evidence that suppressive antiviral therapy with acyclovir, valacyclovir or famciclovir in pacients experiencing at least four recurrences of genital herpes per year decreases the number of pacients with at least one recurrence as compared with placebo. Network meta-analysis of the few direct comparisons and the indirect comparisons did not show superiority of one drug over another.
Conflict of interest statement
L Le Cleach, L. Trinquart, Giao Do, B Lebrun‐Vignes and Philippe Ravaud have no conflicts of interest to declare
O Chosidow was a consultant for GSK.
Figures





































Comment in
-
ACP Journal Club: review: oral antiviral drugs reduce clinical recurrence in recurrent genital herpes.Ann Intern Med. 2015 Feb 17;162(4):JC7. doi: 10.7326/ACPJC-2015-162-4-007. Ann Intern Med. 2015. PMID: 25686194 No abstract available.
References
References to studies included in this review
Blom 1986 {published data only (unpublished sought but not used)}
-
- Blom I, Back O, Egelrud T, Frodin T, Hellbe L, Johannisson G, et al. Long‐term oral acyclovir treatment prevents recurrent genital herpes. Dermatologica 1986; Vol. 173, issue 5:220‐3. - PubMed
Diaz‐Mitoma 1998 {published data only (unpublished sought but not used)}
-
- Diaz‐Mitoma F, Sibbald R G, Shafran S D, Boon R, Saltzman R L. Oral famciclovir for the suppression of recurrent genital herpes: a randomized controlled trial. Collaborative Famciclovir Genital Herpes Research Group. JAMA 1998; Vol. 280, issue 10:887‐92. - PubMed
Douglas 1984 {published data only (unpublished sought but not used)}
-
- Douglas J M, Critchlow C, Benedetti J, Mertz G J, Connor J D, Hintz M A, et al. A double‐blind study of oral acyclovir for suppression of recurrences of genital herpes simplex virus infection. New England Journal of Medicine 1984; Vol. 310, issue 24:1551‐6. - PubMed
Fife 2006 {published and unpublished data}
-
- HS2100273. A randomized , double‐blind, placebo‐controlled,multicenter 60‐day study comparing the efficacy of valtrex 1gr once daily vs placebo once daily in reducing viral shedding in immunocompetent subjects with recurrent HSV_2 genital herpes. gsk‐clinicalstudyregister.com 2005.
-
- Fife KH, Warren TJ, Ferrera RD, Young DG, Justus SE, Heitman CK, et al. Effect of valacyclovir on viral shedding in immunocompetent patients with recurrent herpes simplex virus 2 genital herpes: a US‐based randomized, double‐blind, placebo‐controlled clinical trial. Mayo Clinic Proceedings. Mayo Clinic 2006; Vol. 81, issue 10:1321‐7. - PubMed
Halsos 1985 {published data only}
-
- Halsos AM, Gundersen T, Tjotta EAL. Suppression of recurrent genital herpes by oral application of acyclovir. H+G Zeitschrift fur Hautkrankheiten 1985;60(22):1817‐8. - PubMed
-
- Halsos AM, Salo OP, Lassus A, Tjotta EA, Hovi T, Gabrielsen BO, et al. Oral acyclovir suppression of recurrent genital herpes: a double‐blind, placebo‐controlled, crossover study. Acta Dermato‐Venereologica 1985;65(1):59‐63. - PubMed
HS240017 2005 {unpublished data only}
-
- A multicenter , double blind, placebo‐controlled trial to evaluate daily suppressive therapy with Valtrex on the rate of HSV shedding in subject with recurrent genital herpes. gsk‐clinicalstudyregister.com 2005.
Kinghorn 1985 {published data only (unpublished sought but not used)}
Kinghorn 1992 {published data only (unpublished sought but not used)}
Kroon 1989 {published data only (unpublished sought but not used)}
-
- Kroon S, Petersen CS, Andersen LP, Rasmussen LP, Vestergaard BF. Oral acyclovir suppressive therapy in severe recurrent genital herpes. A double‐blind, placebo‐controlled cross‐over study. Danish Medical Bulletin 1989; Vol. 36, issue 3:298‐300. - PubMed
Mattison 1988 {published data only (unpublished sought but not used)}
-
- Clive D, Corey L, Reichman RC, Davis LG, Hozier JC. A double‐blind, placebo‐controlled cytogenetic study of oral acyclovir in patients with recurrent genital herpes. Journal of Infectious Diseases 1991;164(4):753‐7. - PubMed
-
- Mattison HR, Reichman RC, Benedetti J, Bolgiano D, Davis LG, Bailey‐Farchione A, et al. Double‐blind, placebo‐controlled trial comparing long‐term suppressive with short‐term oral acyclovir therapy for management of recurrent genital herpes. American Journal of Medicine 1988; Vol. 85, issue 2A:20‐5. - PubMed
Mertz 1988 {published data only (unpublished sought but not used)}
-
- Baker DA. Long‐term suppressive therapy with acyclovir for recurrent genital herpes. Journal of International Medical Research 1994;22 Suppl 1:24A‐31A; discussion 31A‐32. - PubMed
-
- Baker DA, Blythe JG, Kaufman R, Hale R, Portnoy J. One‐year suppression of frequent recurrences of genital herpes with oral acyclovir. Obstetrics and Gynecology 1989;73(1):84‐7. - PubMed
-
- Erlich KS, Hauer L, Mills J. Effects of long‐term acyclovir chemosuppression on serum IgG antibody to herpes simplex virus. Journal of Medical Virology 1988;26(1):33‐9. - PubMed
-
- Fife KH, Crumpacker CS, Mertz GJ, Hill EL, Boone GS. Recurrence and resistance patterns of herpes simplex virus following cessation of > or = 6 years of chronic suppression with acyclovir. Acyclovir Study Group. Journal of Infectious Diseases 1994;169(6):1338‐41. - PubMed
-
- Goldberg LH, Kaufman R, Kurtz TO, Conant MA, Eron L J, Batenhorst RL, et al. Long‐term suppression of recurrent genital herpes with acyclovir. A 5‐year benchmark. Acyclovir Study Group. Archives of Dermatology 1993; Vol. 129, issue 5:582‐7. - PubMed
Mertz 1997 {published data only}
-
- Mertz GJ, Loveless MO, Levin MJ, Kraus SJ, Fowler SL, Goade D, et al. Oral famciclovir for suppression of recurrent genital herpes simplex virus infection in women. A multicenter, double‐blind, placebo‐controlled trial. Collaborative Famciclovir Genital Herpes Research Group. Archives of Internal Medicine 1997; Vol. 157, issue 3:343‐9. - PubMed
Mindel 1984 {published data only (unpublished sought but not used)}
-
- Mindel A, Weller IV, Faherty A, Sutherland S, Hindley D, Fiddian AP, et al. Prophylactic oral acyclovir in recurrent genital herpes. Lancet 1984; Vol. 2, issue 8394:57‐9. - PubMed
Mostow 1988 {published data only}
-
- Mostow SR, Mayfield JL, Marr JJ, Drucker JL. Suppression of recurrent genital herpes by single daily dosages of acyclovir. American Journal of Medicine 1988; Vol. 85, issue 2A:30‐3. - PubMed
Patel 1997 {published and unpublished data}
-
- 123‐037. gsk‐clinicalstudyregister.com 2005.
-
- Patel R, Bodsworth NJ, Woolley P, Peters B, Vejlsgaard G, Saari S, et al. Valaciclovir for the suppression of recurrent genital HSV infection: a placebo controlled study of once daily therapy. International Valaciclovir HSV Study Group. Genitourinary Medicine 1997; Vol. 73, issue 2:105‐9. - PMC - PubMed
PREV123 2006 {unpublished data only}
-
- Multicentre randomised trial comparing two strategies for the episodic use of valacyclovir , alone or combined with suppressive treatment in immunocompetent patients with recurrent genital herpes. gsk‐clinicalstudyregister.com 2006.
Reitano 1998 {published and unpublished data}
-
- 123‐026. gsk‐clinicalstudyregister.com 2005.
-
- Reitano M, Tyring S, Lang W, Thoming C, Worm A M, Borelli S, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large‐scale dose range‐finding study. International Valaciclovir HSV Study Group. Journal of Infectious Diseases 1998; Vol. 178, issue 3:603‐10. - PubMed
Romanowski 2003 {published data only (unpublished sought but not used)}
-
- Romanowski B, Marina R B, Roberts J N. Patients' preference of valacyclovir once‐daily suppressive therapy versus twice‐daily episodic therapy for recurrent genital herpes: a randomized study. Sexually Transmitted Diseases 2003;30(3):226‐31. - PubMed
-
- Taback NA, Bradley C. Validation of the genital herpes treatment satisfaction questionnaire (GHerpTSQ) in status and change versions. Quality of Life Research 2006; Vol. 15, issue 6:1043‐52. - PubMed
Sacks 1988 {published data only (unpublished sought but not used)}
-
- Sacks SL, Fox R, Levendusky P, Stiver HG, Roland S, Nusinoff‐Lehrman S, et al. Chronic suppression for six months compared with intermittent lesional therapy of recurrent genital herpes using oral acyclovir: effects on lesions and nonlesional prodromes. Sexually Transmitted Diseases 1988; Vol. 15, issue 1:58‐62. - PubMed
Sacks 2004 {published data only (unpublished sought but not used)}
-
- Sacks SL. Famciclovir suppression of asymptomatic and symptomatic recurrent anogenital herpes simplex virus shedding in women: a randomized, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, single‐center trial. Journal of Infectious Diseases 2004; Vol. 189, issue 8:1341‐7. - PubMed
Sekhin 2004 {published data only}
-
- Sekhin SV, Averchenkov NA, Petrochenkova, N Melekhova Y, Stratchounkski LS. Efficacy of long‐term suppressive therapy with valacyclovir in recurrent genital herpes. Clinical Microbiology and Infection 2004;10(Suppl 3):43‐44.
Straus 1984 {published data only (unpublished sought but not used)}
-
- Straus SE, Seidlin M, Takiff HE, BachrachS, DiGiovanna JJ, Western KA, et al. Suppression of recurrent genital herpes with oral acyclovir. Transactions of the Association of American Physicians 1983; Vol. 96:278‐83. - PubMed
-
- Straus SE, Takiff HE, Seidlin M, Bachrach S, Lininger L, DiGiovanna JJ, et al. Suppression of frequently recurring genital herpes. A placebo‐controlled double‐blind trial of oral acyclovir. New England journal of medicine 1984; Vol. 310, issue 24:1545‐50. - PubMed
Thin 1985 {published data only (unpublished sought but not used)}
-
- Thin R N, Jeffries DJ, Taylor PK, Ayra OP, Rodin P, Yeo J, et al. Recurrent genital herpes suppressed by oral acyclovir: a multicentre double blind trial. Journal of Antimicrobial Chemotherapy 1985; Vol. 16, issue 2:219‐26. - PubMed
Velasco 1991 {published data only (unpublished sought but not used)}
-
- Velasco M, Saavedra T, Sepulveda C, Suarez M. [Prolonged treatment with acyclovir in recurrent genital herpes. Clinical, virological, and immunological response]. Revista Medica de Chile 1991; Vol. 119, issue 8:876‐80. - PubMed
Wald 2006 study 1 {published data only (unpublished sought but not used)}
-
- Wald A, Selke S, Warren T, Aoki FY, Sacks S, Diaz‐Mitoma F, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sexually Transmitted Diseases 2006; Vol. 33, issue 9:529‐33. - PubMed
Wald 2006 study 2 {published data only (unpublished sought but not used)}
-
- Wald A, Selke S, Warren T, Aoki FY, Sacks S, Diaz‐Mitoma F, et al. Comparative efficacy of famciclovir and valacyclovir for suppression of recurrent genital herpes and viral shedding. Sexually Transmitted Diseases 2006;33(9):529‐33. - PubMed
References to studies excluded from this review
Bartlett 2008 {published and unpublished data}
-
- CFAM 810AU07. Novartis clinical trial database (www.novctrd.com).
-
- Bartlett BL, Tyring SK, Fife K, Gnann JW Jr, Hadala JT, Kianifard F, et al. Famciclovir treatment options for patients with frequent outbreaks of recurrent genital herpes: the RELIEF trial. Journal of Clinical Virology 2008; Vol. 43, issue 2:190‐5. - PubMed
Celum 2008 {published data only}
-
- Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delany‐Moretlwe S, et al. Effect of aciclovir on HIV‐1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double‐blind, placebo‐controlled trial. Lancet 2008; Vol. 371, issue 9630:2109‐19. - PMC - PubMed
Corey 2004b {published data only}
-
- Corey L, Wald A, Patel R, Sacks S, Tyring SK, Warren T, et al. Once‐daily valacyclovir to reduce the risk of transmission of genital herpes. New England Journal of Medicine 2004; Vol. 350, issue 1:11‐20. - PubMed
-
- Kim HN, Wald A, Harris J, Almekinder J, Heitman C, Corey L. Does frequency of genital herpes recurrences predict risk of transmission? Further analysis of the valacyclovir transmission study. Sexually Transmitted Diseases 2008; Vol. 35, issue 2:124‐8. - PubMed
Fife 2007 {published data only (unpublished sought but not used)}
-
- Fife KH, Almekinder J, Ofner S. A comparison of one year of episodic or suppressive treatment of recurrent genital herpes with valacyclovir. Sexually Transmitted Diseases 2007; Vol. 34, issue 5:297‐301. - PubMed
Fuchs 2010 {published data only}
Gold 1988 {published data only}
-
- Gold D, Ashley R, Solberg G, Abbo H, Corey L. Chronic‐dose acyclovir to suppress frequently recurring genital herpes simplex virus infection: effect on antibody response to herpes simplex virus type 2 proteins. Journal of Infectious Diseases 1988; Vol. 158, issue 6:1227‐34. - PubMed
Gupta 2004 {published data only}
-
- Gupta R, Wald A, Krantz E, Selke S, Warren T, Vargas‐Cortes M, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. Journal of Infectious Diseases 2004; Vol. 190, issue 8:1374‐81. - PubMed
HS240018 {unpublished data only}
-
- A randomized, double‐blind, multicenter study of valtrex 500mg suppressive therapy in the reduction of anxiety associated with recurrent genital herpes. gsk‐clinicalstudyregister.com. [GSK databases]
HS240021 {unpublished data only}
-
- A randomized, double‐blind, multicenter study of Valtrex 500mg Suppressive therapy in the reduction of anxiety associated with recurrent genital herpes. gsk‐clinicalstudyregister.com. [GSK databases]
Johnston 2011 {published data only}
-
- Johnston C, Saracino M, Kuntz S, Magaret A, Schiffer JT, Selke S, et al. Frequent breakthrough genital HSV‐2 shedding on standard and high dose valacyclovir. Sexually Transmitted Infections 2011;87:A79.
Johnston 2012 {published data only}
Leone 2007 {published data only}
-
- Leone P, Warren T, Hamed K, Fife K, Wald A. Famciclovir reduces viral mucosal shedding in HSV‐seropositive persons. Sexually Transmitted Diseases 2007; Vol. 34, issue 11:900‐7. - PubMed
Mindel 1988 {published data only}
-
- Mindel A, Faherty A, Carney O, Patou G, Freris M, Williams P. Dosage and safety of long‐term suppressive acyclovir therapy for recurrent genital herpes. Lancet 1988; Vol. 1, issue 8591:926‐8. - PubMed
Mindel 1989 {published data only}
Schiffer 2011 {published data only}
Strachan 2011 {published data only}
-
- Strachan E, Saracino M, Selke S, Magaret A, Buchwald D, Wald A. The effects of daily distress and personality on genital HSV shedding and lesions in a randomized, double‐blind, placebo‐controlled, crossover trial of acyclovir in HSV‐2 seropositive women. Brain, Behavior, and Immunity 2011; Vol. 25, issue 7:1475‐81. - PMC - PubMed
Straus 1986 {published data only}
-
- Straus SE, Seidlin M, Takiff HE, Rooney J F, Lehrman S N, Bachrach S, et al. Double‐blind comparison of weekend and daily regimens of oral acyclovir for suppression of recurrent genital herpes. Antiviral Research 1986; Vol. 6, issue 3:151‐9. - PubMed
Wald 1996 {published data only}
-
- Gupta R, Hill EL, McClernon D, Davis G, Selke S, Corey L, et al. Acyclovir sensitivity of sequential herpes simplex virus type 2 isolates from the genital mucosa of immunocompetent women. Journal of Infectious Diseases 2005; Vol. 192, issue 6:1102‐7. - PubMed
-
- Wald A, Zeh J, Barnum G, Davis L G, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Annals of Internal Medicine 1996;124(1 Pt 1):8‐15. - PubMed
References to studies awaiting assessment
Douglas 1988 {published data only (unpublished sought but not used)}
-
- Douglas JM Jr, Davis LG, Remington ML, Paulsen CA, Perrin EB, Goodman P, et al. A double‐blind, placebo‐controlled trial of the effect of chronically administered oral acyclovir on sperm production in men with frequently recurrent genital herpes. Journal of Infectious Diseases 1988; Vol. 157, issue 3:588‐93. - PubMed
Frenkel 1989 {published data only (unpublished sought but not used)}
-
- Frenkel L, Pineda E, Garratty E, Fall H, Dillon M, Bryson Y. A prospective study of the effects of acyclovir treatment on the HSV‐2 lymphoproliferative response of persons with frequently recurring HSV‐2 genital infections. Journal of Infectious Diseases 1989; Vol. 159, issue 5:845‐50. - PubMed
Mastrolorenzo 1991 {published data only}
-
- Mastrolorenzo A, Zuccati G, Tiradritti L, Brandini L, Filippeschi C, Panconesi E. [Treatment of recurrent herpes simplex genitalis]. Giornale Italiano di Chemioterapia 1991; Vol. 38, issue 1‐3:61‐2. - PubMed
Tyring 2003 {published data only}
-
- Tyring SK, Diaz‐Mitoma F, Shafran SD, Locke LA, Sacks SL, Young CL. Oral famciclovir for the suppression of recurrent genital herpes: the combined data from two randomized controlled trials. Journal of Cutaneous Medicine and Surgery 2003; Vol. 7, issue 6:449‐54. - PubMed
Wu 2000 {published data only}
-
- Wu WZ. A randomized controlled clinical study on valaciclovir in the treatment of recurrent genital herpes in different course of treatment. Chinese Journal of Antibiotics 2000;25(6):475‐7.
Zhang 2011 {published data only}
-
- Zhang L, Yang HL, Liu ZR, Fan JY, Xuan M, Long CQ, Bi JJ. To comparative the effect of aciclovir and valaciclovir on preventing the recurrence of recurrent genital herpes. Journal of Clinical Dermatology 2011;40(4):213.
References to ongoing studies
NCT01658826 {unpublished data only}
-
- Safety and efficacy comparator trial of a new drug against genital herpes. Ongoing study October 2012.
Additional references
Abudalu 2008
-
- Abudalu M, Tyring S, Koltun W, Bodsworth N, Hamed K. Single‐day, patient‐initiated famciclovir therapy versus 3‐day valacyclovir regimen for recurrent genital herpes: a randomized, double‐blind, comparative trial. Clinical Infectious Diseases 2008;47(5):651‐8. [PUBMED: 18637755] - PubMed
Aoki 2006
-
- Aoki FY, Tyring S, Diaz‐Mitoma F, Gross G, Gao J, Hamed K. Single‐day, patient‐initiated famciclovir therapy for recurrent genital herpes: a randomized, double‐blind, placebo‐controlled trial. Clinical Infectious Diseases 2006;42(1):8‐13. [PUBMED: 16323085] - PubMed
Benedetti 1994
-
- Benedetti J, Corey L, Ashley R. Recurrence rates in genital herpes after symptomatic first‐episode infection. Annals of Internal Medicine 1994;121(11):847‐54. [PUBMED: 7978697] - PubMed
Bodsworth 2009
-
- Bodsworth N, Fife K, Koltun W, Tyring S, Abudalu M, Prichard M, et al. Single‐day famciclovir for the treatment of genital herpes: follow‐up results of time to next recurrence and assessment of antiviral resistance. Current Medical Research and Opinion 2009;25(2):483‐7. [PUBMED: 19192993] - PMC - PubMed
Celum 2004
Cochrane Handbook
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2014]. The Cochrane Collaboration,, 2011.
Corey 2004
-
- Corey L, Wald A, Patel R, Sacks SL, Tyring SK, Warren T, et al. Once‐daily valacyclovir to reduce the risk of transmission of genital herpes. New England Journal of Medicine 2004;350(1):11‐20. [PUBMED: 14702423] - PubMed
Curtin 2002
-
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Stat Med 2002;21(15):2145‐59. - PubMed
De Clercq 2006
Engelberg 2003
-
- Engelberg R, Carrell D, Krantz E, Corey L, Wald A. Natural history of genital herpes simplex virus type 1 infection. Sexually Transmitted Diseases 2003;30(2):174‐7. [PUBMED: 12567178] - PubMed
European guidelines (IUSTI)
-
- Patel R, Alderson S, Geretti A, et al. 2010 European guideline for management of genital herpes. http://www.iusti.org/regions/Europe/euroguidelines.htm (accessed September 2010).
Freeman 2006
-
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta‐analysis of longitudinal studies. AIDS (London, England) 2006;20(1):73‐83. [PUBMED: 16327322] - PubMed
Goldberg 1986
-
- Goldberg LH, Kaufman R, Conant MA, Sperber J, Allen ML, Illeman M, et al. Oral acyclovir for episodic treatment of recurrent genital herpes. Efficacy and safety. Journal of the American Academy of Dermatology 1986;15(2 Pt 1):256‐64. [PUBMED: 3745529] - PubMed
Gupta 2007
-
- Gupta R, Warren T, Wald A. Genital herpes. Lancet 2007;370(9605):2127‐37. [PUBMED: 18156035] - PubMed
Hollier 2008
-
- Hollier LM, Wendel GD. Third trimester antiviral prophylaxis for preventing maternal genital herpes simplex virus (HSV) recurrences and neonatal infection. Cochrane Database of Systematic Reviews (Online) 2008;1:CD004946. [PUBMED: 18254066] - PubMed
Ioannidis 2007
Ioannidis 2007a
Kimberlin 2004
-
- Kimberlin DW, Rouse DJ. Clinical practice. Genital herpes. New England Journal of Medicine 2004;350(19):1970‐7. [PUBMED: 15128897] - PubMed
Kimberlin 2013
Lebrun‐Vignes 2007
-
- Lebrun‐Vignes B, Bouzamondo A, Dupuy A, Guillaume JC, Lechat P, Chosidow O. A meta‐analysis to assess the efficacy of oral antiviral treatment to prevent genital herpes outbreaks. Journal of the American Academy of Dermatology 2007;57(2):238‐46. [PUBMED: 17416440] - PubMed
Leone 2002
-
- Leone PA, Trottier S, Miller JM. Valacyclovir for episodic treatment of genital herpes: a shorter 3‐day treatment course compared with 5‐day treatment. Clinical Infectious Diseases 2002;34(7):958‐62. [PUBMED: 11880962] - PubMed
Looker 2008
Lu 2004
-
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23(20):3105‐24. [PUBMED: 15449338] - PubMed
Martin 2009
MMWR 2010
-
- CDC. Seroprevalence of herpes simplex virus type 2 among persons aged 14‐49 years‐‐United States, 2005‐2008. MMWR. Morbidity and Mortality Weekly Report 2010;59(15):456‐9. [PUBMED: 20414188] - PubMed
Moreno 2009
Reichman 1984
-
- Reichman RC, Badger GJ, Mertz GJ, Corey L, Richman DD, Connor JD, et al. Treatment of recurrent genital herpes simplex infections with oral acyclovir. A controlled trial. JAMA 1984;251(16):2103‐7. [PUBMED: 6368877] - PubMed
Romanowski 2000
-
- Romanowski B, Aoki FY, Martel AY, Lavender EA, Parsons JE, Saltzman RL. Efficacy and safety of famciclovir for treating mucocutaneous herpes simplex infection in HIV‐infected individuals. Collaborative Famciclovir HIV Study Group. AIDS (London, England) 2000;14(9):1211‐7. [PUBMED: 10894286] - PubMed
Rosenberry 1982
-
- Rosenberry KR, Bryan CK, Sohn CA. Acyclovir: evaluation of a new antiviral agent. Clinical Pharmacy 1982;1(5):399‐406. [PUBMED: 6307582] - PubMed
Rucker 2011
-
- Rucker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics (Oxford, England) 2011;12(1):122‐42. [PUBMED: 20656692] - PubMed
Sacks 2005
-
- Sacks SL, Aoki FY, Martel AY, Shafran SD, Lassonde M. Clinic‐initiated, twice‐daily oral famciclovir for treatment of recurrent genital herpes: a randomized, double‐blind, controlled trial. Clinical Infectious Diseases 2005;41(8):1097‐104. [PUBMED: 16163627] - PubMed
Saiag 1999
-
- Saiag P, Praindhui D, Chastang C. A double‐blind, randomized study assessing the equivalence of valacyclovir 1000 mg once daily versus 500 mg twice daily in the episodic treatment of recurrent genital herpes. Genival Study Group. Journal of Antimicrobial Chemotherapy 1999;44(4):525‐31. [PUBMED: 10588314] - PubMed
Salanti 2010
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [PUBMED: 20688472] - PubMed
Scoular 2002
Sen 2007
Slomka 1998
-
- Slomka MJ, Emery L, Munday PE, Moulsdale M, Brown DW. A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. Journal of medical virology 1998;55(2):177‐83. [PUBMED: 9598940] - PubMed
Tran 2012
Trinquart 2012
Tronstein 2011
Tyring 1998
-
- Tyring SK, Douglas JM Jr, Corey L, Spruance SL, Esmann J. A randomized, placebo‐controlled comparison of oral valacyclovir and acyclovir in immunocompetent patients with recurrent genital herpes infections. The Valaciclovir International Study Group. Archives of Dermatology 1998;134(2):185‐91. [PUBMED: 9487210] - PubMed
Wald 2002
-
- Wald A, Carrell D, Remington M, Kexel E, Zeh J, Corey L. Two‐day regimen of acyclovir for treatment of recurrent genital herpes simplex virus type 2 infection. Clinical Infectious Diseases 2002;34(7):944‐8. [PUBMED: 11880960] - PubMed
Warren 2004
-
- Warren T, Harris J, Brennan CA. Efficacy and safety of valacyclovir for the suppression and episodic treatment of herpes simplex virus in patients with HIV. Clinical Infectious Diseases 2004;39 Suppl 5:S258‐66. [PUBMED: 15494897] - PubMed
Whitley 2001
-
- Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet 2001;357(9267):1513‐8. [PUBMED: 11377626] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

