A monoclonal antibody-based immunoassay for measuring the potency of 2009 pandemic influenza H1N1 vaccines
- PMID: 25087462
- PMCID: PMC4181825
- DOI: 10.1111/irv.12272
A monoclonal antibody-based immunoassay for measuring the potency of 2009 pandemic influenza H1N1 vaccines
Abstract
Background: The potency of inactivated influenza vaccines is determined using a single radial immunodiffusion (SRID) assay. This assay is relatively easy to standardize, it is not technically demanding, and it is capable of measuring the potency of several vaccine strain subtypes in a multivalent vaccine. Nevertheless, alternative methods that retain the major advantages of the SRID, but with a greater dynamic range of measurement and with reduced reagent requirements, are needed.
Objectives: The feasibility of an ELISA-based assay format was explored as an alternative potency assay for inactivated influenza vaccines.
Methods: Several murine monoclonal antibodies (mAbs), specific for the 2009 pandemic H1N1 influenza virus hemagglutinin (HA), were evaluated for their potential to capture and quantify HA antigen. Vaccine samples, obtained from four licensed influenza vaccine manufacturers, included monovalent bulk vaccine, monovalent vaccine, and trivalent vaccine. Traditional SRID potency assays were run in parallel with the mAb-ELISA potency assay using the reference antigen standard appropriate for the vaccine samples being tested.
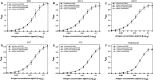
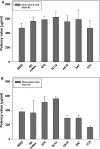
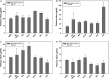
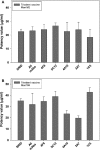
Results: The results indicated that the ELISA potency assay can quantify HA over a wide range of concentrations, including vaccine at subpotent doses, and the ELISA and SRID potency values correlated well for most vaccine samples. Importantly, the assay was capable of quantifying A/California HA in a trivalent formulation.
Conclusions: This study demonstrates the general feasibility of the mAb approach and strongly suggests that such ELISAs have potential for continued development as an alternative method to assay the potency of inactivated influenza vaccines.
Keywords: Influenza virus vaccine; monoclonal antibody; potency assay.
© 2014 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Potency determination of inactivated H7 influenza vaccines using monoclonal antibody-based ELISA and biolayer interferometry assays.Influenza Other Respir Viruses. 2018 Mar;12(2):250-258. doi: 10.1111/irv.12528. Epub 2017 Dec 15. Influenza Other Respir Viruses. 2018. PMID: 29152878 Free PMC article.
-
Standardisation of inactivated influenza vaccines-Learning from history.Influenza Other Respir Viruses. 2018 Mar;12(2):195-201. doi: 10.1111/irv.12543. Epub 2018 Feb 2. Influenza Other Respir Viruses. 2018. PMID: 29356318 Free PMC article. Review.
-
Determination of influenza B identity and potency in quadrivalent inactivated influenza vaccines using lineage-specific monoclonal antibodies.PLoS One. 2017 Apr 19;12(4):e0175733. doi: 10.1371/journal.pone.0175733. eCollection 2017. PLoS One. 2017. PMID: 28423025 Free PMC article.
-
An alternative method for preparation of pandemic influenza strain-specific antibody for vaccine potency determination.Vaccine. 2010 Mar 11;28(12):2442-9. doi: 10.1016/j.vaccine.2009.12.079. Epub 2010 Jan 12. Vaccine. 2010. PMID: 20074687
-
Critical review of current and emerging quantification methods for the development of influenza vaccine candidates.Vaccine. 2015 Nov 4;33(44):5913-9. doi: 10.1016/j.vaccine.2015.07.104. Epub 2015 Aug 10. Vaccine. 2015. PMID: 26271833 Review.
Cited by
-
Hemagglutinin Quantitative ELISA-based Potency Assay for Trivalent Seasonal Influenza Vaccine Using Group-Specific Universal Monoclonal Antibodies.Sci Rep. 2019 Dec 23;9(1):19675. doi: 10.1038/s41598-019-56169-5. Sci Rep. 2019. PMID: 31873147 Free PMC article.
-
Assaying the Potency of Influenza Vaccines.Vaccines (Basel). 2015 Feb 5;3(1):90-104. doi: 10.3390/vaccines3010090. Vaccines (Basel). 2015. PMID: 26344948 Free PMC article. Review.
-
Development of an ELISA-Based Potency Assay for Inactivated Influenza Vaccines Using Cross-Reactive Nanobodies.Vaccines (Basel). 2022 Sep 5;10(9):1473. doi: 10.3390/vaccines10091473. Vaccines (Basel). 2022. PMID: 36146550 Free PMC article.
-
Potency determination of inactivated H7 influenza vaccines using monoclonal antibody-based ELISA and biolayer interferometry assays.Influenza Other Respir Viruses. 2018 Mar;12(2):250-258. doi: 10.1111/irv.12528. Epub 2017 Dec 15. Influenza Other Respir Viruses. 2018. PMID: 29152878 Free PMC article.
-
Standardisation of inactivated influenza vaccines-Learning from history.Influenza Other Respir Viruses. 2018 Mar;12(2):195-201. doi: 10.1111/irv.12543. Epub 2018 Feb 2. Influenza Other Respir Viruses. 2018. PMID: 29356318 Free PMC article. Review.
References
-
- Wood JM, Schild GC, Newman RW, Seagroatt V. An improved single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen: application for potency determinations of inactivated whole virus and subunit vaccines. J Biol Stand. 1977;5:237–247. - PubMed
-
- Williams MS. Single-radial-immunodiffusion as an in vitro potency assay for human inactivated viral vaccines. Vet Microbiol. 1993;37:253–262. - PubMed
-
- Minor PD, Engelhardt OG, Wood JM, et al. Current challenges in implementing cell-derived influenza vaccines: implications for production and regulation, July 2007, NIBSC, Potters Bar, UK. Vaccine. 2009;27:2907–2913. - PubMed
-
- Cate TR, Couch RB, Parker D, Baxter B. Reactogenicity, immunogenicity, and antibody persistence in adults given inactivated influenza virus vaccines – 1978. Rev Infect Dis. 1983;5:737–747. - PubMed
-
- La Montagne JR, Noble GR, Quinnan GV, et al. Summary of clinical trials of inactivated influenza vaccine – 1978. Rev Infect Dis. 1983;5:723–736. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

