Automated monitoring and quantitative analysis of feeding behaviour in Drosophila
- PMID: 25087594
- PMCID: PMC4143931
- DOI: 10.1038/ncomms5560
Automated monitoring and quantitative analysis of feeding behaviour in Drosophila
Abstract
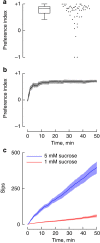
Food ingestion is one of the defining behaviours of all animals, but its quantification and analysis remain challenging. This is especially the case for feeding behaviour in small, genetically tractable animals such as Drosophila melanogaster. Here, we present a method based on capacitive measurements, which allows the detailed, automated and high-throughput quantification of feeding behaviour. Using this method, we were able to measure the volume ingested in single sips of an individual, and monitor the absorption of food with high temporal resolution. We demonstrate that flies ingest food by rhythmically extending their proboscis with a frequency that is not modulated by the internal state of the animal. Instead, hunger and satiety homeostatically modulate the microstructure of feeding. These results highlight similarities of food intake regulation between insects, rodents, and humans, pointing to a common strategy in how the nervous systems of different animals control food intake.
Figures




References
-
- Simpson S. J. & Raubenheimer D. The Nature of Nutrition: A Unifying Framework From Animal Adaptation To Human Obesity Princeton University Press (2012).
-
- Ribeiro C. & Dickson B. J. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 20, 1000–1005 (2010). - PubMed
-
- Zinke I., Kirchner C., Chao L. C., Tetzlaff M. T. & Pankratz M. J. Suppression of food intake and growth by amino acids in Drosophila: the role of pumpless, a fat body expressed gene with homology to vertebrate glycine cleavage system. Development 126, 5275–5284 (1999). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

