Ursolic acid ameliorates autoimmune arthritis via suppression of Th17 and B cell differentiation
- PMID: 25087995
- PMCID: PMC4155530
- DOI: 10.1038/aps.2014.58
Ursolic acid ameliorates autoimmune arthritis via suppression of Th17 and B cell differentiation
Abstract
Aim: Ursolic acid (UA) is a pentacyclic triterpenoid found in most plant species, which has been shown anti-inflammatory and anti-oxidative activities. In this study, we examined the effects of UA on collagen-induced arthritis (CIA) in mice, and to identify the mechanisms underlying the effects.
Methods: CIA was induced in mice. Two weeks later, the mice were treated with UA (150 mg/kg, ip, 3 times per week) for 4 weeks. The expression of cytokines and oxidative stress markers in joint tissues was measured with immunohistochemistry. The numbers of CD4+IL-17+, CD4+CD25+Foxp3+ and pSTAT3 cells in spleens were determined using confocal immunostaining or flowcytometric analyses. Serum antibody levels and B cell-associated marker mRNAs were analyzed with ELISAs and qRT-PCR, respectively. CD4+ T cells and CD19+ B cells were purified from mice spleens for in vitro studies.
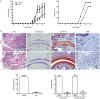
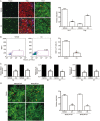
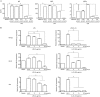
Results: UA treatment significantly reduced the incidence and severity of CIA-induced arthritis, accompanied by decreased expression of proinflammatory cytokines (TNF-α, IL-1β, IL-6, IL-21 and IL-17) and oxidative stress markers (nitrotyrosine and iNOS) in arthritic joints. In CIA mice, UA treatment significantly decreased the number of Th17 cells, while increased the number of Treg cells in the spleens, which was consistent with decreased expression of pSTAT3, along with IL-17 and RORγt in the splenocytes. In addition, UA treatment significantly reduced the serum CII-specific IgG levels in CIA mice. The inhibitory effects of UA on Th17 cells were confirmed in an in vitro model of Th17 differentiation. Furthermore, UA dose-dependently suppressed the expression of B cell-associated markers Bcl-6, Blimp1 and AID mRNAs in purified CD19+ B cells pretreated with IL-21 or LPS in vitro.
Conclusion: UA treatment significantly ameliorates CIA in mice via suppression of Th17 and differentiation. By targeting pathogenic Th17 cells and autoantibody production, UA may be useful for the treatment of autoimmune arthritis and other Th17-related diseases.
Figures



References
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. New Engl J Med. 2011;365:2205–19. - PubMed
-
- Egan PJ, van Nieuwenhuijze A, Campbell IK, Wicks IP. Promotion of the local differentiation of murine Th17 cells by synovial macrophages during acute inflammatory arthritis. Arthritis Rheum. 2008;58:3720–9. - PubMed
-
- Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK, et al. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol. 2003;170:2655–62. - PubMed
-
- Zuniga LA, Jain R, Haines C, Cua DJ. Th17 cell development: from the cradle to the grave. Immunol Rev. 2013;252:78–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

