Single-cell phenotyping within transparent intact tissue through whole-body clearing
- PMID: 25088144
- PMCID: PMC4153367
- DOI: 10.1016/j.cell.2014.07.017
Single-cell phenotyping within transparent intact tissue through whole-body clearing
Abstract
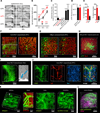
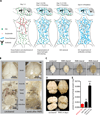
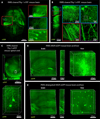
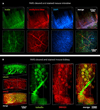
Understanding the structure-function relationships at cellular, circuit, and organ-wide scale requires 3D anatomical and phenotypical maps, currently unavailable for many organs across species. At the root of this knowledge gap is the absence of a method that enables whole-organ imaging. Herein, we present techniques for tissue clearing in which whole organs and bodies are rendered macromolecule-permeable and optically transparent, thereby exposing their cellular structure with intact connectivity. We describe PACT (passive clarity technique), a protocol for passive tissue clearing and immunostaining of intact organs; RIMS (refractive index matching solution), a mounting media for imaging thick tissue; and PARS (perfusion-assisted agent release in situ), a method for whole-body clearing and immunolabeling. We show that in rodents PACT, RIMS, and PARS are compatible with endogenous-fluorescence, immunohistochemistry, RNA single-molecule FISH, long-term storage, and microscopy with cellular and subcellular resolution. These methods are applicable for high-resolution, high-content mapping and phenotyping of normal and pathological elements within intact organs and bodies.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Becker K, Jahrling N, Saghafi S, Dodt HU. Ultramicroscopy: light-sheet-based microscopy for imaging centimeter-sized objects with micrometer resolution. Cold Spring Harbor protocols. 2013;2013:704–713. - PubMed
-
- Chung K, Deisseroth K. CLARITY for mapping the nervous system. Nature methods. 2013;10:508–513. - PubMed
Publication types
MeSH terms
Grants and funding
- T32 GM007616/GM/NIGMS NIH HHS/United States
- T32 GM008042/GM/NIGMS NIH HHS/United States
- R21 MH103824/MH/NIMH NIH HHS/United States
- 5T32GM07616/GM/NIGMS NIH HHS/United States
- R01 NS085910/NS/NINDS NIH HHS/United States
- 1R21MH103824-01/MH/NIMH NIH HHS/United States
- DP2 NS087949/NS/NINDS NIH HHS/United States
- R01 HD075605/HD/NICHD NIH HHS/United States
- 5T32AR058921/AR/NIAMS NIH HHS/United States
- R01 AG047664/AG/NIA NIH HHS/United States
- R01HD075605/HD/NICHD NIH HHS/United States
- 1R01NS085910-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

