Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion
- PMID: 25088414
- PMCID: PMC4138044
- DOI: 10.1016/j.celrep.2014.06.060
Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion
Abstract
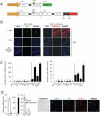
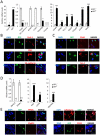
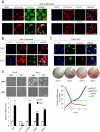
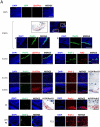
Pref-1 is an EGF-repeat-containing protein that inhibits adipocyte differentiation. To better understand the origin and development of white adipose tissue (WAT), we generated transgenic mouse models for transient or permanent fluorescent labeling of cells using the Pref-1 promoter, facilitating inducible ablation. We show that Pref-1-marked cells retain proliferative capacity and are very early adipose precursors, prior to expression of Zfp423 or PPARγ. In addition, the Pref-1-marked cells establish that adipose precursors are mesenchymal, but not endothelial or pericytal, in origin. During embryogenesis, Pref-1-marked cells first appear in the dorsal mesenteric region as early as embryonic day 10.5 (E10.5). These cells become lipid-laden adipocytes at E17.5 in the subcutaneous region, whereas visceral WAT develops after birth. Finally, ablation of Pref-1-marked cells prevents not only embryonic WAT development but also later adult adipose expansion upon high-fat feeding, demonstrating the requirement of Pref-1 cells for adipogenesis.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Billon N, Dani C. The generation of adipocytes by the neural crest. Stem Cell Rev. 2012;8:55–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

