Focused ultrasound-mediated non-invasive brain stimulation: examination of sonication parameters
- PMID: 25088462
- PMCID: PMC4167941
- DOI: 10.1016/j.brs.2014.06.011
Focused ultrasound-mediated non-invasive brain stimulation: examination of sonication parameters
Abstract
Background: Transcranial focused ultrasound (FUS) has emerged as a new brain stimulation modality. The range of sonication parameters for successful brain stimulation warrants further investigation.
Objective: The objective of this study was to examine the range of FUS sonication parameters that minimize the acoustic intensity/energy deposition while successfully stimulating the motor brain area in Sprague-Dawley rats.
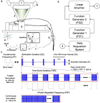
Methods: We transcranially administered FUS to the somatomotor area of the rat brain and measured the acoustic intensity that caused excitatory effects with respect to different pulsing parameters (tone-burst duration, pulse-repetition frequency, duty cycle, and sonication duration) at 350 and 650 kHz of fundamental frequency.
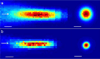
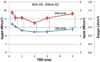
Results: We observed that motor responses were elicited at minimum threshold acoustic intensities (4.9-5.6 W/cm(2) in spatial-peak pulse-average intensity; 2.5-2.8 W/cm(2) in spatial-peak temporal-average intensity) in a limited range of sonication parameters, i.e. 1-5 ms of tone-burst duration, 50% of duty cycle, and 300 ms of sonication duration, at 350 kHz fundamental frequency. We also found that the pulsed sonication elicited motor responses at lower acoustic intensities than its equivalent continuous sonication.
Conclusion: Our results suggest that the pulsed application of FUS selectively stimulates specific brain areas-of-interest at an acoustic intensity that is compatible with regulatory safety limits on biological tissue, thus allowing for potential applications in neurotherapeutics.
Keywords: Focused ultrasound; Neuromodulation; Neurostimulation; Parameter; Sonication.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Suppression of EEG visual-evoked potentials in rats through neuromodulatory focused ultrasound.Neuroreport. 2015 Mar 4;26(4):211-5. doi: 10.1097/WNR.0000000000000330. Neuroreport. 2015. PMID: 25646585 Free PMC article.
-
Estimation of the spatial profile of neuromodulation and the temporal latency in motor responses induced by focused ultrasound brain stimulation.Neuroreport. 2014 May 7;25(7):475-9. doi: 10.1097/WNR.0000000000000118. Neuroreport. 2014. PMID: 24384503 Free PMC article.
-
Focused ultrasound-mediated noninvasive blood-brain barrier modulation: preclinical examination of efficacy and safety in various sonication parameters.Neurosurg Focus. 2018 Feb;44(2):E15. doi: 10.3171/2017.11.FOCUS17627. Neurosurg Focus. 2018. PMID: 29385915
-
Low-intensity ultrasound neuromodulation: An overview of mechanisms and emerging human applications.Brain Stimul. 2018 Nov-Dec;11(6):1209-1217. doi: 10.1016/j.brs.2018.08.013. Epub 2018 Aug 23. Brain Stimul. 2018. PMID: 30166265 Review.
-
Safety Review and Perspectives of Transcranial Focused Ultrasound Brain Stimulation.Brain Neurorehabil. 2021 Mar 17;14(1):e4. doi: 10.12786/bn.2021.14.e4. eCollection 2021 Mar. Brain Neurorehabil. 2021. PMID: 36742103 Free PMC article. Review.
Cited by
-
A practical approach to the ethical use of memory modulating technologies.BMC Med Ethics. 2020 Sep 18;21(1):89. doi: 10.1186/s12910-020-00532-z. BMC Med Ethics. 2020. PMID: 32948166 Free PMC article.
-
On the Neuromodulatory Pathways of the In Vivo Brain by Means of Transcranial Focused Ultrasound.Curr Opin Biomed Eng. 2018 Dec;8:61-69. doi: 10.1016/j.cobme.2018.10.004. Epub 2018 Nov 15. Curr Opin Biomed Eng. 2018. PMID: 31223668 Free PMC article.
-
Advances in magnetic field approaches for non-invasive targeting neuromodulation.Front Hum Neurosci. 2025 Apr 28;19:1489940. doi: 10.3389/fnhum.2025.1489940. eCollection 2025. Front Hum Neurosci. 2025. PMID: 40356879 Free PMC article. Review.
-
Hydrogels in wearable neural interfaces.Med X. 2024;2(1):23. doi: 10.1007/s44258-024-00040-4. Epub 2024 Dec 9. Med X. 2024. PMID: 39659711 Free PMC article. Review.
-
Simultaneous acoustic stimulation of human primary and secondary somatosensory cortices using transcranial focused ultrasound.BMC Neurosci. 2016 Oct 26;17(1):68. doi: 10.1186/s12868-016-0303-6. BMC Neurosci. 2016. PMID: 27784293 Free PMC article.
References
-
- Ettinger GJ, Leventon ME, Grimson WE, Kikinis R, Gugino L, Cote W, et al. Experimentation with a transcranial magnetic stimulation system for functional brain mapping. Medical image analysis. 1998 Jun;2(2):133–142. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, Non-P.H.S.]. - PubMed
-
- Picht T, Schmidt S, Brandt S, Frey D, Hannula H, Neuvonen T, et al. Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery. 2011 Sep;69(3):581–588. [Comparative Study]. - PubMed
-
- Mancuso JJ, Kim J, Lee S, Tsuda S, Chow NB, Augustine GJ. Optogenetic probing of functional brain circuitry. Experimental physiology. 2011 Jan;96(1):26–33. [Review]. - PubMed
-
- McDannold NJ, Jolesz FA, Hynynen KH. Determination of the Optimal Delay between Sonications during Focused Ultrasound Surgery in Rabbits by Using MR Imaging to Monitor Thermal Buildup in Vivo1. Radiology. 1999;211(2):419–426. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

