MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion
- PMID: 25088770
- PMCID: PMC4148706
- DOI: 10.1016/j.immuni.2014.06.016
MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion
Abstract
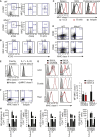
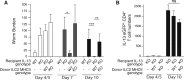
Group 2 innate lymphoid cells (ILC2s) release interleukin-13 (IL-13) during protective immunity to helminth infection and detrimentally during allergy and asthma. Using two mouse models to deplete ILC2s in vivo, we demonstrate that T helper 2 (Th2) cell responses are impaired in the absence of ILC2s. We show that MHCII-expressing ILC2s interact with antigen-specific T cells to instigate a dialog in which IL-2 production from T cells promotes ILC2 proliferation and IL-13 production. Deletion of MHCII renders IL-13-expressing ILC2s incapable of efficiently inducing Nippostrongylus brasiliensis expulsion. Thus, during transition to adaptive T cell-mediated immunity, the ILC2 and T cell crosstalk contributes to their mutual maintenance, expansion and cytokine production. This interaction appears to augment dendritic-cell-induced T cell activation and identifies a previously unappreciated pathway in the regulation of type-2 immunity.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
MHC-II: a mutual support system for ILCs and T cells?Immunity. 2014 Aug 21;41(2):174-6. doi: 10.1016/j.immuni.2014.07.006. Immunity. 2014. PMID: 25148019
References
-
- Barlow J.L., Bellosi A., Hardman C.S., Drynan L.F., Wong S.H., Cruickshank J.P., McKenzie A.N. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J. Allergy Clin. Immunol. 2012;129:191–198. e191-194. - PubMed
-
- Barnden M.J., Allison J., Heath W.R., Carbone F.R. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 1998;76:34–40. - PubMed
-
- Chan C.W., Crafton E., Fan H.N., Flook J., Yoshimura K., Skarica M., Brockstedt D., Dubensky T.W., Stins M.F., Lanier L.L. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat. Med. 2006;12:207–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

