THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg (THAWS) Trial
- PMID: 25088843
- PMCID: PMC4660886
- DOI: 10.1111/ijs.12360
THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg (THAWS) Trial
Abstract
Rationale: Because of lack of information regarding timing of stroke, patients who suffer stroke during sleep are generally ineligible for intravenous thrombolysis, although many of these patients could potentially recover with this treatment. Magnetic resonance image findings with positive diffusion-weighted imaging and no marked parenchymal hyperintensity on fluid-attenuated inversion recovery (negative pattern) can identify acute ischemic stroke patients within 4·5 h from symptom onset.
Aims: The THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0·6 mg/kg trial aims to determine the efficacy and safety of intravenous thrombolysis with alteplase at 0·6 mg/kg body weight, the approved dose for Japanese stroke patients, using magnetic resonance image-based selection in ischemic stroke patients with unclear time of symptom onset, and compare findings with standard treatment.
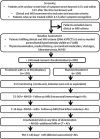
Design: This is an investigator-initiated, multicenter, prospective, randomized, open-treatment, blinded-end-point clinical trial. The design is similar to the Efficacy and Safety of MRI-based Thrombolysis in Wake-up Stroke trial. Patients with unclear-onset time of stroke symptoms beyond 4·5 h and within 12 h after the time of the last-known-well period and within 4·5 h after symptom recognition, who showed a negative fluid-attenuated inversion recovery pattern, are randomized to either intravenous thrombolysis or standard treatment.
Study outcomes: The primary efficacy end-point is modified Rankin Scale 0-1 at 90 days. The safety outcome measures are symptomatic intracranial hemorrhage at 22-36 h, and major bleeding and mortality at 90 days.
Discussion: This trial may help determine if low-dose alteplase at 0·6 mg/kg should be recommended as a routine clinical strategy for ischemic stroke patients with unclear-onset time.
Trial registration: ClinicalTrials.gov NCT02002325.
Keywords: acute ischemic stroke; clinical trials; diffusion-weighted imaging; fluid-attenuated inversion recovery imaging; thrombolysis; unclear-onset time.
© 2014 World Stroke Organization.
Figures

References
-
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. - PubMed
-
- Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. - PubMed
-
- Kang DW, Kwon JY, Kwon SU, Kim JS. Wake-up or unclear-onset strokes: are they waking up to the world of thrombolysis therapy? Int J Stroke. 2012;7:311–320. - PubMed
-
- Koton S, Tanne D, Bornstein NM. Ischemic stroke on awakening: patients' characteristics, outcomes and potential for reperfusion therapy. Neuroepidemiology. 2012;39:149–153. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

