Innovative agents in multiple myeloma
- PMID: 25089218
- PMCID: PMC4114494
Innovative agents in multiple myeloma
Abstract
Multiple myeloma (MM) remains an incurable cancer of the bone marrow plasma cells. However, the overall survival of patients with MM has increased dramatically within the past decade. This is due, in part, to newer agents such as immunomodulatory drugs (lenalidomide, thalidomide, and pomalidomide) and proteasome inhibitors (bortezomib, carfilzomib, MLN9708). These and several other new classes of drugs have arisen from an improved understanding of the complex environment in which genetic changes occur. Improved understanding of genetic events will enable clinicians to better stratify risk before and during therapy, tailor treatment, and test the value of personalized interventions. The ultimate goal in this incurable disease setting is to reduce the impact of cancer- or chemotherapy-related side effects. Nurses and advanced practitioners are integral to the treatment team. Thus, each should be aware of changes to the current drug landscape. Targeted drugs with sophisticated mechanisms of action are currently under investigation. Patients gain access to newer drugs within the context of clinical trials. Awareness of such trials will help accrual and determine if therapeutic benefit exists. In this article, we will describe new agents with unique and targeted mechanisms of action that have activity in patients with relapsed and/or refractory multiple myeloma.
Figures
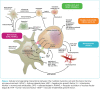

References
-
- Bensinger William, Maziarz Richard T, Jagannath Sundar, Spencer Andrew, Durrant Simon, Becker Pamela S, Ewald Brett, Bilic Sanela, Rediske John, Baeck Johan, Stadtmauer Edward A. A phase 1 study of lucatumumab, a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. British journal of haematology. 2012;159:58–66. - PubMed
-
- Berdeja J., Savona M., Mace J., Hart L., Essell J., Owera R., Flinn I. A single-arm, open-label, multi-center phase I/II study of the combination of panobinostat and carfilzomib in patients (pts) with relapsed or relapse/refractory multiple myeloma (MM) [Abstract 1937]. Blood (ASH Annual Meeting Abstracts) 2013;122
-
- Berenson James R, Hilger James D, Yellin Ori, Boccia Ralph V, Matous Jeffrey, Dressler Kenneth, Ghazal Hassan H, Jamshed Saad, Kingsley Edwin C, Harb Wael A, Noga Stephen J, Nassir Youram, Swift Regina A, Vescio Robert. A phase 1/2 study of oral panobinostat combined with melphalan for patients with relapsed or refractory multiple myeloma. Annals of hematology. 2014;93:89–98. - PubMed
-
- Bolduc Anna, Long Eugene, Stapler Dale, Cascalho Marilia, Tsubata Takeshi, Koni Pandelakis A, Shimoda Michiko. Constitutive CD40L expression on B cells prematurely terminates germinal center response and leads to augmented plasma cell production in T cell areas. Journal of immunology (Baltimore, Md. : 1950) 2010;185:220–230. - PMC - PubMed
-
- Dimopoulos M. A., Jagannath S., Yoon S.-S., Siegel D. S., Lonial S., Hajek R., Anderson K. C. Vantage 088: Vorinostat in combination with bortezomib in patients with relapsed/refractory multiple myeloma: Results of a global, randomized phase 3 trial [Abstract 811] Blood (ASH Annual Meeting Abstracts) 2011
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
