Two β-galactosidases from the human isolate Bifidobacterium breve DSM 20213: molecular cloning and expression, biochemical characterization and synthesis of galacto-oligosaccharides
- PMID: 25089712
- PMCID: PMC4121272
- DOI: 10.1371/journal.pone.0104056
Two β-galactosidases from the human isolate Bifidobacterium breve DSM 20213: molecular cloning and expression, biochemical characterization and synthesis of galacto-oligosaccharides
Abstract
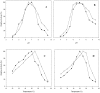
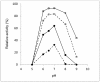
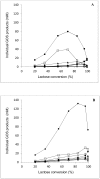
Two β-galactosidases, β-gal I and β-gal II, from Bifidobacterium breve DSM 20213, which was isolated from the intestine of an infant, were overexpressed in Escherichia coli with co-expression of the chaperones GroEL/GroES, purified to electrophoretic homogeneity and biochemically characterized. Both β-gal I and β-gal II belong to glycoside hydrolase family 2 and are homodimers with native molecular masses of 220 and 211 kDa, respectively. The optimum pH and temperature for hydrolysis of the two substrates o-nitrophenyl-β-D-galactopyranoside (oNPG) and lactose were determined at pH 7.0 and 50°C for β-gal I, and at pH 6.5 and 55°C for β-gal II, respectively. The kcat/Km values for oNPG and lactose hydrolysis are 722 and 7.4 mM-1s-1 for β-gal I, and 543 and 25 mM-1s-1 for β-gal II. Both β-gal I and β-gal II are only moderately inhibited by their reaction products D-galactose and D-glucose. Both enzymes were found to be very well suited for the production of galacto-oligosaccharides with total GOS yields of 33% and 44% of total sugars obtained with β-gal I and β-gal II, respectively. The predominant transgalactosylation products are β-D-Galp-(1→6)-D-Glc (allolactose) and β-D-Galp-(1→3)-D-Lac, accounting together for more than 75% and 65% of the GOS formed by transgalactosylation by β-gal I and β-gal II, respectively, indicating that both enzymes have a propensity to synthesize β-(1→6) and β-(1→3)-linked GOS. The resulting GOS mixtures contained relatively high fractions of allolactose, which results from the fact that glucose is a far better acceptor for galactosyl transfer than galactose and lactose, and intramolecular transgalactosylation contributes significantly to the formation of this disaccharide.
Conflict of interest statement
Figures


References
-
- Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr 125: 1401–1412. - PubMed
-
- Bouhnik Y, Raskine L, Simoneau G, Vicaut E, Neut C, et al. (2004) The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: A double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J ClinNutr 80: 1658–1664. - PubMed
-
- Biavati B, Mattarelli P (2006) The family Bifidobacteriaceae. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E, editors. The prokaryotes. 3rd ed. New York: Springer. pp. 322–382.
-
- Tanaka R (1995) Clinical effects of bifidobacteria and lactobacilli. In: Fuller R, Heidt PJ, Rusch V, Waaij DVD, editors. Probiotics: prospects of use in opportunistic infections Old Herborn University seminar monograph 8: Institute for Microbiology and Biochemistry, Herborn-Dill, Germany. pp. 141–157.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

