The changing burden of hepatitis C virus infection in the United States: model-based predictions
- PMID: 25089861
- PMCID: PMC4356484
- DOI: 10.7326/M14-0095
The changing burden of hepatitis C virus infection in the United States: model-based predictions
Abstract
Background: Chronic hepatitis C virus (HCV) infection causes a substantial health and economic burden in the United States. With the availability of direct-acting antiviral agents, recently approved therapies and those under development, and 1-time birth-cohort screening, the burden of this disease is expected to decrease.
Objective: To predict the effect of new therapies and screening on chronic HCV infection and associated disease outcomes.
Design: Individual-level state-transition model.
Setting: Existing and anticipated therapies and screening for HCV infection in the United States.
Patients: Total HCV-infected population in the United States.
Measurements: The number of cases of chronic HCV infection and outcomes of advanced-stage HCV infection.
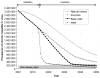
Results: The number of cases of chronic HCV infection decreased from 3.2 million in 2001 to 2.3 million in 2013. One-time birth-cohort screening beginning in 2013 is expected to identify 487,000 cases of HCV infection in the next 10 years. In contrast, 1-time universal screening could identify 933,700 cases. With the availability of highly effective therapies, HCV infection could become a rare disease in the next 22 years. Recently approved therapies for HCV infection and 1-time birth-cohort screening could prevent approximately 124,200 cases of decompensated cirrhosis, 78,800 cases of hepatocellular carcinoma, 126,500 liver-related deaths, and 9900 liver transplantations by 2050. Increasing the treatment capacity would further reduce the burden of HCV disease.
Limitation: Institutionalized patients with HCV infection were excluded, and empirical data on the effectiveness of future therapies and on the future annual incidence and treatment capacity of HCV infection are lacking.
Conclusion: New therapies for HCV infection and widespread implementation of screening and treatment will play an important role in reducing the burden of HCV disease. More aggressive screening recommendations are needed to identify a large pool of infected patients.
Primary funding source: National Institutes of Health.
Figures

References
-
- Rosen HR. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429–38. - PubMed
-
- Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–8. - PubMed
-
- Drenth JP. HCV Treatment—No More Room for Interferonologists? N Engl J Med. 2013;368(20):1931–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
