Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial
- PMID: 25090173
- PMCID: PMC4811188
- DOI: 10.1001/jamainternmed.2014.2556
Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial
Abstract
Importance: Buprenorphine opioid agonist treatment (OAT) has established efficacy for treating opioid dependency among persons seeking addiction treatment. However, effectiveness for out-of-treatment, hospitalized patients is not known.
Objective: To determine whether buprenorphine administration during medical hospitalization and linkage to office-based buprenorphine OAT after discharge increase entry into office-based OAT, increase sustained engagement in OAT, and decrease illicit opioid use at 6 months after hospitalization.
Design, setting, and participants: From August 1, 2009, through October 31, 2012, a total of 663 hospitalized, opioid-dependent patients in a general medical hospital were identified. Of these, 369 did not meet eligibility criteria. A total of 145 eligible patients consented to participation in the randomized clinical trial. Of these, 139 completed the baseline interview and were assigned to the detoxification (n = 67) or linkage (n = 72) group.
Interventions: Five-day buprenorphine detoxification protocol or buprenorphine induction, intrahospital dose stabilization, and postdischarge transition to maintenance buprenorphine OAT affiliated with the hospital's primary care clinic (linkage).
Main outcomes and measures: Entry and sustained engagement with buprenorphine OAT at 1, 3, and 6 months (medical record verified) and prior 30-day use of illicit opioids (self-report).
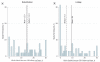
Results: During follow-up, linkage participants were more likely to enter buprenorphine OAT than those in the detoxification group (52 [72.2%] vs 8 [11.9%], P < .001). At 6 months, 12 linkage participants (16.7%) and 2 detoxification participants (3.0%) were receiving buprenorphine OAT (P = .007). Compared with those in the detoxification group, participants randomized to the linkage group reported less illicit opioid use in the 30 days before the 6-month interview (incidence rate ratio, 0.60; 95% CI, 0.46-0.73; P < .01) in an intent-to-treat analysis.
Conclusions and relevance: Compared with an inpatient detoxification protocol, initiation of and linkage to buprenorphine treatment is an effective means for engaging medically hospitalized patients who are not seeking addiction treatment and reduces illicit opioid use 6 months after hospitalization. However, maintaining engagement in treatment remains a challenge.
Trial registration: clinicaltrials.gov Identifier: NCT00987961.
Figures
Comment in
-
Improving care for hospitalized, opioid-dependent patients: a promising start.JAMA Intern Med. 2014 Aug;174(8):1377-8. doi: 10.1001/jamainternmed.2014.728. JAMA Intern Med. 2014. PMID: 24978013 No abstract available.
References
-
- Brown RL, Leonard T, Saunders LA, Papasouliotis O. The prevalence and detection of substance use disorders among inpatients ages 18 to 49: an opportunity for prevention. Prev Med. 1998;27(1):101–110. doi:10.1006/pmed.1997.0250. - PubMed
-
- Moore RD, Bone LR, Geller G, Mamon JA, Stokes EJ, Levine DM. Prevalence, detection, and treatment of alcoholism in hospitalized patients. JAMA. 1989;261(3):403–407. - PubMed
-
- Stein MD, Wilkinson J, Berglas N, O’Sullivan P. Prevalence and detection of illicit drug disorders among hospitalized patients. Am J Drug Alcohol Abuse. 1996;22(3):463–471. - PubMed
-
- Katz A, Goldberg D, Smith J, Trick WE. Tobacco, alcohol, and drug use among hospital patients: concurrent use and willingness to change. J Hosp Med. 2008;3(5):369–375. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

