Long-term physiological alterations and recovery in a mouse model of separation associated with time-restricted feeding: a tool to study anorexia nervosa related consequences
- PMID: 25090643
- PMCID: PMC4121212
- DOI: 10.1371/journal.pone.0103775
Long-term physiological alterations and recovery in a mouse model of separation associated with time-restricted feeding: a tool to study anorexia nervosa related consequences
Abstract
Background: Anorexia nervosa is a primary psychiatric disorder, with non-negligible rates of mortality and morbidity. Some of the related alterations could participate in a vicious cycle limiting the recovery. Animal models mimicking various physiological alterations related to anorexia nervosa are necessary to provide better strategies of treatment.
Aim: To explore physiological alterations and recovery in a long-term mouse model mimicking numerous consequences of severe anorexia nervosa.
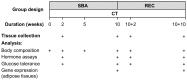
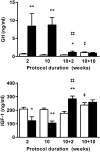
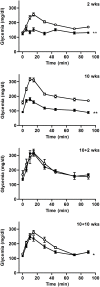
Methods: C57Bl/6 female mice were submitted to a separation-based anorexia protocol combining separation and time-restricted feeding for 10 weeks. Thereafter, mice were housed in standard conditions for 10 weeks. Body weight, food intake, body composition, plasma levels of leptin, adiponectin, IGF-1, blood levels of GH, reproductive function and glucose tolerance were followed. Gene expression of several markers of lipid and energy metabolism was assayed in adipose tissues.
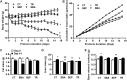
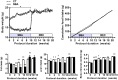
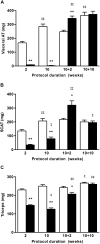
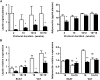
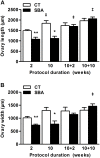
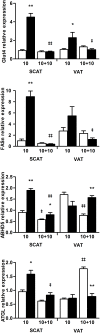
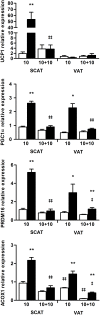
Results: Mimicking what is observed in anorexia nervosa patients, and despite a food intake close to that of control mice, separation-based anorexia mice displayed marked alterations in body weight, fat mass, lean mass, bone mass acquisition, reproductive function, GH/IGF-1 axis, and leptinemia. mRNA levels of markers of lipogenesis, lipolysis, and the brown-like adipocyte lineage in subcutaneous adipose tissue were also changed. All these alterations were corrected during the recovery phase, except for the hypoleptinemia that persisted despite the full recovery of fat mass.
Conclusion: This study strongly supports the separation-based anorexia protocol as a valuable model of long-term negative energy balance state that closely mimics various symptoms observed in anorexia nervosa, including metabolic adaptations. Interestingly, during a recovery phase, mice showed a high capacity to normalize these parameters with the exception of plasma leptin levels. It will be interesting therefore to explore further the central and peripheral effects of the uncorrected hypoleptinemia during recovery from separation-based anorexia.
Conflict of interest statement
Figures
References
-
- Hoek HW (2006) Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr Opin Psychiatry 19: 389–394. - PubMed
-
- Legroux-Gérot I, Vignau J, D’Herbomez M, Collier F, Marchandise X, et al. (2007) Evaluation of bone loss and mechanisms in anorexia nervosa. Calcif Tissue Int 81: 174–182. - PubMed
-
- Zipfel S, Seibel MJ, Löwe B, Beumont PJ, Kasperk C, et al. (2001) Osteoporosis in eating disorders: a follow-up study of patients with anorexia and bulimia nervosa. J Clin Endocrinol Metab 86: 5227–5233. - PubMed
-
- Legroux-Gérot I, Vignau J, Biver E, Pigny P, Collier F, et al. (2010) Anorexia nervosa, osteoporosis and circulating leptin: the missing link. Osteoporos Int 21: 1715–1722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

