Identification of a new androgen receptor (AR) co-regulator BUD31 and related peptides to suppress wild-type and mutated AR-mediated prostate cancer growth via peptide screening and X-ray structure analysis
- PMID: 25091737
- PMCID: PMC4253602
- DOI: 10.1016/j.molonc.2014.06.009
Identification of a new androgen receptor (AR) co-regulator BUD31 and related peptides to suppress wild-type and mutated AR-mediated prostate cancer growth via peptide screening and X-ray structure analysis
Abstract
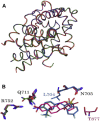
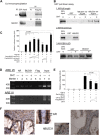
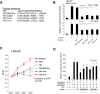
Treatment with individual anti-androgens is associated with the development of hot-spot mutations in the androgen receptor (AR). Here, we found that anti-androgens-mt-ARs have similar binary structure to the 5α-dihydrotestosterone-wt-AR. Phage display revealed that these ARs bound to similar peptides, including BUD31, containing an Fxx(F/H/L/W/Y)Y motif cluster with Tyr in the +5 position. Structural analyses of the AR-LBD-BUD31 complex revealed formation of an extra hydrogen bond between the Tyr+5 residue of the peptide and the AR. Functional studies showed that BUD31-related peptides suppressed AR transactivation, interrupted AR N-C interaction, and suppressed AR-mediated cell growth. Combination of peptide screening and X-ray structure analysis may serve as a new strategy for developing anti-ARs that simultaneously suppress both wt and mutated AR function.
Keywords: Androgen receptor; Anti-androgen withdrawal syndrome; BUD31; Crystallography; FxxLF.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
New Insights into the Binding Mechanism of Co-regulator BUD31 to AR AF2 Site: Structural Determination and Analysis of the Mutation Effect.Curr Comput Aided Drug Des. 2020;16(1):45-53. doi: 10.2174/1573409915666190502153307. Curr Comput Aided Drug Des. 2020. PMID: 31057123 Free PMC article.
-
Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone.J Biol Chem. 2007 Aug 31;282(35):25801-16. doi: 10.1074/jbc.M703268200. Epub 2007 Jun 25. J Biol Chem. 2007. PMID: 17591767 Free PMC article.
-
Structural features discriminate androgen receptor N/C terminal and coactivator interactions.Mol Cell Endocrinol. 2012 Jan 30;348(2):403-10. doi: 10.1016/j.mce.2011.03.026. Epub 2011 Jun 1. Mol Cell Endocrinol. 2012. PMID: 21664945 Free PMC article. Review.
-
Androgen receptor (AR) NH2- and COOH-terminal interactions result in the differential influences on the AR-mediated transactivation and cell growth.Mol Endocrinol. 2005 Feb;19(2):350-61. doi: 10.1210/me.2004-0190. Epub 2004 Oct 28. Mol Endocrinol. 2005. PMID: 15514032
-
Peptide antagonist of the androgen receptor.Curr Pharm Des. 2010;16(9):1106-13. doi: 10.2174/138161210790963850. Curr Pharm Des. 2010. PMID: 20030610 Review.
Cited by
-
Gene Regulation Network Analysis on Human Prostate Orthografts Highlights a Potential Role for the JMJD6 Regulon in Clinical Prostate Cancer.Cancers (Basel). 2021 Apr 26;13(9):2094. doi: 10.3390/cancers13092094. Cancers (Basel). 2021. PMID: 33925994 Free PMC article.
-
A partially open conformation of an androgen receptor ligand-binding domain with drug-resistance mutations.Acta Crystallogr F Struct Biol Commun. 2023 Apr 1;79(Pt 4):95-104. doi: 10.1107/S2053230X23002224. Epub 2023 Mar 30. Acta Crystallogr F Struct Biol Commun. 2023. PMID: 36995121 Free PMC article.
-
The role of BUD31 in clear cell renal cell carcinoma: prognostic significance, alternative splicing, and tumor immune environment.Clin Exp Med. 2024 Aug 13;24(1):191. doi: 10.1007/s10238-024-01451-8. Clin Exp Med. 2024. PMID: 39136845 Free PMC article.
-
Prostate Cancer Epigenetic Plasticity and Enhancer Heterogeneity: Molecular Causes, Consequences and Clinical Implications.Adv Exp Med Biol. 2022;1390:255-275. doi: 10.1007/978-3-031-11836-4_15. Adv Exp Med Biol. 2022. PMID: 36107324 Review.
-
A network-based pharmacological study on the mechanism of action of muscone in breast cancer.Transl Cancer Res. 2022 May;11(5):1195-1206. doi: 10.21037/tcr-22-667. Transl Cancer Res. 2022. PMID: 35706803 Free PMC article.
References
-
- Akakura, K. , Akimoto, S. , Furuya, Y. , Ito, H. , 1998. Incidence and characteristics of antiandrogen withdrawal syndrome in prostate cancer after treatment with chlormadinone acetate. Eur. Urol.. 33, 567–571. - PubMed
-
- Andersen, R.J. , Mawji, N.R. , Wang, J. , Wang, G. , Haile, S. , Myung, J.K. , Watt, K. , Tam, T. , Yang, Y.C. , Banuelos, C.A. , Williams, D.E. , McEwan, I.J. , Wang, Y. , Sadar, M.D. , 2010. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 17, 535–546. - PubMed
-
- Axerio-Cilies, P. , Lack, N.A. , Nayana, M.R. , Chan, K.H. , Yeung, A. , Leblanc, E. , Guns, E.S. , Rennie, P.S. , Cherkasov, A. , 2011. Inhibitors of androgen receptor activation function-2 (AF2) site identified through virtual screening. J. Med. Chem.. 54, 6197–6205. - PubMed
-
- Bissada, N.K. , Kaczmarek, A.T. , 1995. Complete remission of hormone refractory adenocarcinoma of the prostate in response to withdrawal of diethylstilbestrol. J. Urol.. 153, 1944–1945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

