Nitric oxide exerts protective effects against bleomycin-induced pulmonary fibrosis in mice
- PMID: 25092105
- PMCID: PMC4237963
- DOI: 10.1186/s12931-014-0092-3
Nitric oxide exerts protective effects against bleomycin-induced pulmonary fibrosis in mice
Abstract
Background: Increased expression of nitric oxide synthase (NOS) and an increase in plasma nitrite plus nitrate (NOx) have been reported in patients with pulmonary fibrosis, suggesting that nitric oxide (NO) plays an important role in its development. However, the roles of the entire NO and NOS system in the pathogenesis of pulmonary fibrosis still remain to be fully elucidated. The aim of the present study is to clarify the roles of NO and the NOS system in pulmonary fibrosis by using the mice lacking all three NOS isoforms.
Methods: Wild-type, single NOS knockout and triple NOS knockout (n/i/eNOS-/-) mice were administered bleomycin (BLM) intraperitoneally at a dose of 8.0 mg/kg/day for 10 consecutive days. Two weeks after the end of the procedure, the fibrotic and inflammatory changes of the lung were evaluated. In addition, we evaluated the effects of long-term treatment with isosorbide dinitrate, a NO donor, on the n/i/eNOS-/- mice with BLM-induced pulmonary fibrosis.
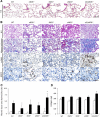
Results: The histopathological findings, collagen content and the total cell number in bronchoalveolar lavage fluid were the most severe/highest in the n/i/eNOS-/- mice. Long-term treatment with the supplemental NO donor in n/i/eNOS-/- mice significantly prevented the progression of the histopathological findings and the increase of the collagen content in the lungs.
Conclusions: These results provide the first direct evidence that a lack of all three NOS isoforms led to a deterioration of pulmonary fibrosis in a BLM-treated murine model. We speculate that the entire endogenous NO and NOS system plays an important protective role in the pathogenesis of pulmonary fibrosis.
Figures






References
-
- Tsutsui M, Shimokawa H, Morishita T, Nakashima Y, Yanagihara N. Development of genetically engineered mice lacking all three nitric oxide synthases. J Pharmacol Sci. 2006;102:147–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

