Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system
- PMID: 25092212
- PMCID: PMC4279864
- DOI: 10.2174/1568009614666140804154511
Overview of proteasome inhibitor-based anti-cancer therapies: perspective on bortezomib and second generation proteasome inhibitors versus future generation inhibitors of ubiquitin-proteasome system
Abstract
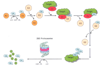
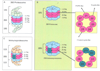
Over the past ten years, proteasome inhibition has emerged as an effective therapeutic strategy for treating multiple myeloma (MM) and some lymphomas. In 2003, Bortezomib (BTZ) became the first proteasome inhibitor approved by the U.S. Food and Drug Administration (FDA). BTZ-based therapies have become a staple for the treatment of MM at all stages of the disease. The survival rate of MM patients has improved significantly since clinical introduction of BTZ and other immunomodulatory drugs. However, BTZ has several limitations. Not all patients respond to BTZ based therapies and relapse occurs in many patients who initially responded. Solid tumors, in particular, are often resistant to BTZ. Furthermore, BTZ can induce dose-limiting peripheral neuropathy (PN). The second generation proteasome inhibitor Carfizomib (CFZ; U.S. FDA approved in August 2012) induces responses in a minority of MM patients relapsed from or refractory to BTZ. There is less PN compared to BTZ. Four other second-generation proteasome inhibitors (Ixazomib, Delanzomib, Oprozomib and Marizomib) with different pharmacologic properties and broader anticancer activities, have also shown some clinical activity in bortezomib-resistant cancers. While the mechanism of resistance to bortezomib in human cancers still remains to be fully understood, targeting the immunoproteasome, ubiquitin E3 ligases, the 19S proteasome and deubiquitinases in pre-clinical studies represents possible directions for future generation inhibitors of ubiquitin-proteasome system in the treatment of MM and other cancers.
Conflict of interest statement
The authors confirm that this article content has no conflict of interest.
Figures
References
-
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79:13–21. - PubMed
-
- Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr. Opin. Cell. Biol. 1995;7:215–223. - PubMed
-
- Goldberg AL, Akopian TR, Kisselev AF, Lee DH, Rohrwild M. New insights into the mechanisms and importance of the proteasome in intracellular protein degradation. Biol. Chem. 1997;378:131–140. - PubMed
-
- Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat. Rev. Drug Discov. 2006;5(7):596–613. - PubMed
-
- Adams J. The proteasome: a suitable antineoplastic target. Nat. Rev. Cancer. 2004;4:349–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

