Rate and determinants of residual viremia in multidrug-experienced patients successfully treated with raltegravir-based regimens
- PMID: 25092266
- PMCID: PMC4287117
- DOI: 10.1089/AID.2014.0060
Rate and determinants of residual viremia in multidrug-experienced patients successfully treated with raltegravir-based regimens
Abstract
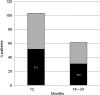
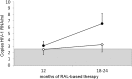
Residual HIV viremia, defined by low levels of plasma HIV RNA with enhanced-sensitivity assays, may persist even in the presence of successful antiretroviral therapy, but little is known about its determinants. Our objective was to evaluate the rate and determinants of residual viremia in patients who show stable undetectable plasma HIV-1 RNA with conventional assays. Forty-four multidrug-experienced patients with undetectable levels of HIV RNA for at least 2 years under raltegravir-based regimens were evaluated. An ultrasensitive (2.5 copies/ml) real-time PCR method was used to quantify plasma HIV RNA. After 12 months of salvage treatment, 48.3% of the patients had residual viremia between 2.5 and 37 copies/ml. The proportion of patients with plasma HIV RNA below 2.5 copies/ml decreased from 51.7% at 12 months to 30.8% at 24 months. The presence of residual viremia was not associated with levels of viremia before starting raltegravir. Considering CD4 counts, hepatitis B or C virus (HBV or HCV) coinfection, or other demographic characteristics, for the time interval between HIV diagnosis and initiation of antiretroviral therapy, patients with a longer interval (>1 year) were significant less likely to have RNA levels below 2.5 copies/ml at 12 months compared to patients who started therapy within 1 year of HIV diagnosis (28.6% vs. 73.3%, p=0.027). Half of the patients showing undetectable HIV viremia with conventional assays had low-level viremia with ultrasensitive assays, with no predictive role of viroimmunological status at the start of the regimen. The potential influence of the interval between HIV diagnosis and initiation of treatment should be confirmed in subjects with a known date of seroconversion.
Figures
References
-
- Havlir DV, Koelsch KK, Strain MC, et al. : Predictors of residual viremia in HIV-infected patients successfully treated with efavirenz and lamivudine plus either tenofovir or stavudine. J Infect Dis 2005;19:1164–1168 - PubMed
-
- Dornadula G, Zhang H, VanUitert B, et al. : Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 1999;282:1627–1632 - PubMed
-
- Palmer S: Advances in detection and monitoring of plasma viremia in HIV-infected individuals receiving antiretroviral therapy. Curr Opin HIV AIDS 2013;8:87–92 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

