Translationally controlled tumor protein is a novel biological target for neurofibromatosis type 1-associated tumors
- PMID: 25092287
- PMCID: PMC4176225
- DOI: 10.1074/jbc.M114.568253
Translationally controlled tumor protein is a novel biological target for neurofibromatosis type 1-associated tumors
Abstract
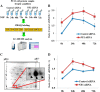
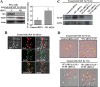
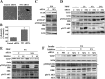
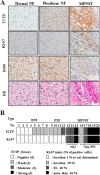
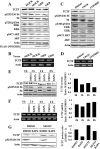
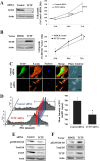
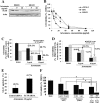
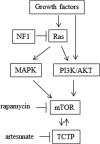
Neurofibromatosis type 1 (NF1) is an autosomal dominant disease that predisposes individuals to develop benign neurofibromas and malignant peripheral nerve sheath tumors (MPNSTs). Due to the lack of information on the molecular mechanism of NF1-associated tumor pathogenesis or biomarkers/therapeutic targets, an effective treatment for NF1 tumors has not been established. In this study, the novel NF1-associated protein, translationally controlled tumor protein (TCTP), was identified by integrated proteomics and found to be up-regulated via activated MAPK/PI3K-AKT signaling in response to growth factors in NF1-deficient Schwann cells. Immunohistochemical analysis of NF1-associated tumors revealed that the TCTP expression level correlated with tumorigenicity. In NF1-deficient MPNST cells, TCTP protein but not mRNA was down-regulated by NF1 GTPase-activating protein-related domain or MAPK/PI3K inhibitors, and this correlated with suppression of mammalian target of rapamycin (mTOR) signaling. mTOR inhibition by rapamycin also down-regulated TCTP protein expression, whereas knockdown or overexpression of TCTP suppressed or activated mTOR signaling, respectively, and affected cell viability. These results suggest that a positive feedback loop between TCTP and mTOR contributes to NF1-associated tumor formation. Last, the anti-tumor effect of artesunate, which binds to and degrades TCTP, was evaluated. Artesunate significantly suppressed the viability of MPNST cells but not normal Schwann cells, and the TCTP level inversely correlated with artesunate sensitivity. Moreover, combinational use of artesunate and rapamycin enhanced the cytotoxic effect on MPNST cells. These findings suggest that TCTP is functionally implicated in the progression of NF1-associated tumors and could serve as a biological target for their therapy.
Keywords: Malignant Peripheral Nerve Sheath Tumors (MPNST); Neurofibroma; Neurofibromatosis Type 1 (NF1); Proteomics; Translationally Controlled Tumor Protein (TCTP); Tumor Marker; Tumor Suppressor Gene; Tumor Therapy; mTOR.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Identification of a Specific Translational Machinery via TCTP-EF1A2 Interaction Regulating NF1-associated Tumor Growth by Affinity Purification and Data-independent Mass Spectrometry Acquisition (AP-DIA).Mol Cell Proteomics. 2019 Feb;18(2):245-262. doi: 10.1074/mcp.RA118.001014. Epub 2018 Oct 31. Mol Cell Proteomics. 2019. PMID: 30381327 Free PMC article.
-
Neurofibromin Deficiency Causes Epidermal Growth Factor Receptor Upregulation through the Activation of Ras/ERK/SP1 Signaling Pathway in Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheet Tumor.Int J Mol Sci. 2021 Dec 10;22(24):13308. doi: 10.3390/ijms222413308. Int J Mol Sci. 2021. PMID: 34948100 Free PMC article.
-
Dual mTORC1/2 inhibition induces anti-proliferative effect in NF1-associated plexiform neurofibroma and malignant peripheral nerve sheath tumor cells.Oncotarget. 2016 Jun 14;7(24):35753-35767. doi: 10.18632/oncotarget.7099. Oncotarget. 2016. PMID: 26840085 Free PMC article.
-
New Model Systems and the Development of Targeted Therapies for the Treatment of Neurofibromatosis Type 1-Associated Malignant Peripheral Nerve Sheath Tumors.Genes (Basel). 2020 Apr 28;11(5):477. doi: 10.3390/genes11050477. Genes (Basel). 2020. PMID: 32353955 Free PMC article. Review.
-
Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors: II. The role of dysregulated growth factor signaling.J Neuropathol Exp Neurol. 2005 Jan;64(1):1-9. doi: 10.1093/jnen/64.1.1. J Neuropathol Exp Neurol. 2005. PMID: 15715079 Review.
Cited by
-
Establishment and genomic characterization of a sporadic malignant peripheral nerve sheath tumor cell line.Sci Rep. 2021 Mar 11;11(1):5690. doi: 10.1038/s41598-021-85055-2. Sci Rep. 2021. PMID: 33707600 Free PMC article.
-
Regulation of epithelial integrity and organ growth by Tctp and Coracle in Drosophila.PLoS Genet. 2020 Jun 19;16(6):e1008885. doi: 10.1371/journal.pgen.1008885. eCollection 2020 Jun. PLoS Genet. 2020. PMID: 32559217 Free PMC article.
-
Progress on the study of the anticancer effects of artesunate.Oncol Lett. 2021 Nov;22(5):750. doi: 10.3892/ol.2021.13011. Epub 2021 Aug 27. Oncol Lett. 2021. PMID: 34539854 Free PMC article. Review.
-
A novel ligand of the translationally controlled tumor protein (TCTP) identified by virtual drug screening for cancer differentiation therapy.Invest New Drugs. 2021 Aug;39(4):914-927. doi: 10.1007/s10637-020-01042-w. Epub 2021 Jan 25. Invest New Drugs. 2021. PMID: 33492639 Free PMC article.
-
Immunohistochemical Localization of Translationally Controlled Tumor Protein in Axon Terminals of Mouse Hippocampal Neurons.Exp Neurobiol. 2017 Apr;26(2):82-89. doi: 10.5607/en.2017.26.2.82. Epub 2017 Mar 7. Exp Neurobiol. 2017. PMID: 28442944 Free PMC article.
References
-
- Stephens K., Riccardi V. M., Rising M., Ng S., Green P., Collins F. S., Rediker K. S., Powers J. A., Parker C., Donis-Keller H. (1987) Linkage studies with chromosome 17 DNA markers in 45 neurofibromatosis 1 families. Genomics 1, 353–357 - PubMed
-
- Cawthon R. M., Weiss R., Xu G. F., Viskochil D., Culver M., Stevens J., Robertson M., Dunn D., Gesteland R., O'Connell P. (1990) A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell 62, 193–201 - PubMed
-
- Costa R. M., Federov N. B., Kogan J. H., Murphy G. G., Stern J., Ohno M., Kucherlapati R., Jacks T., Silva A. J. (2002) Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature 415, 526–530 - PubMed
-
- Yunoue S., Tokuo H., Fukunaga K., Feng L., Ozawa T., Nishi T., Kikuchi A., Hattori S., Kuratsu J., Saya H., Araki N. (2003) Neurofibromatosis type I tumor suppressor neurofibromin regulates neuronal differentiation via its GTPase-activating protein function toward Ras. J. Biol. Chem. 278, 26958–26969 - PubMed
-
- Patrakitkomjorn S., Kobayashi D., Morikawa T., Wilson M. M., Tsubota N., Irie A., Ozawa T., Aoki M., Arimura N., Kaibuchi K., Saya H., Araki N. (2008) Neurofibromatosis type 1 (NF1) tumor suppressor, neurofibromin, regulates the neuronal differentiation of PC12 cells via its associating protein, CRMP-2. J. Biol. Chem. 283, 9399–9413 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

