Protective hinge in insulin opens to enable its receptor engagement
- PMID: 25092300
- PMCID: PMC4143003
- DOI: 10.1073/pnas.1412897111
Protective hinge in insulin opens to enable its receptor engagement
Abstract
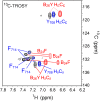
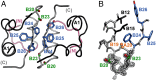
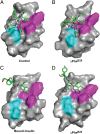
Insulin provides a classical model of a globular protein, yet how the hormone changes conformation to engage its receptor has long been enigmatic. Interest has focused on the C-terminal B-chain segment, critical for protective self-assembly in β cells and receptor binding at target tissues. Insight may be obtained from truncated "microreceptors" that reconstitute the primary hormone-binding site (α-subunit domains L1 and αCT). We demonstrate that, on microreceptor binding, this segment undergoes concerted hinge-like rotation at its B20-B23 β-turn, coupling reorientation of Phe(B24) to a 60° rotation of the B25-B28 β-strand away from the hormone core to lie antiparallel to the receptor's L1-β2 sheet. Opening of this hinge enables conserved nonpolar side chains (Ile(A2), Val(A3), Val(B12), Phe(B24), and Phe(B25)) to engage the receptor. Restraining the hinge by nonstandard mutagenesis preserves native folding but blocks receptor binding, whereas its engineered opening maintains activity at the price of protein instability and nonnative aggregation. Our findings rationalize properties of clinical mutations in the insulin family and provide a previously unidentified foundation for designing therapeutic analogs. We envisage that a switch between free and receptor-bound conformations of insulin evolved as a solution to conflicting structural determinants of biosynthesis and function.
Keywords: diabetes mellitus; metabolism; protein structure; receptor tyrosine kinase; signal transduction.
Conflict of interest statement
Conflict of interest statement: F.I.-B. and M.A.W. hold stock in Thermalin Diabetes, LLC (Cleveland, OH), for which N.B.P. and J.W. are consultants; M.A.W. is Chief Scientific Officer and a Director. Part of M.C.L.'s research is funded by Sanofi (Germany).
Figures
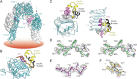





References
-
- Adams MJ, et al. Structure of rhombohedral 2 zinc insulin crystals. Nature. 1969;224(5218):491–495.
-
- Baker EN, et al. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988;319(1195):369–456. - PubMed
-
- Derewenda U, et al. X-ray analysis of the single chain B29-A1 peptide-linked insulin molecule. A completely inactive analogue. J Mol Biol. 1991;220(2):425–433. - PubMed
-
- Mirmira RG, Nakagawa SH, Tager HS. Importance of the character and configuration of residues B24, B25, and B26 in insulin-receptor interactions. J Biol Chem. 1991;266(3):1428–1436. - PubMed
-
- Hua QX, Shoelson SE, Kochoyan M, Weiss MA. Receptor binding redefined by a structural switch in a mutant human insulin. Nature. 1991;354(6350):238–241. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

