Robust efficiency and actuator saturation explain healthy heart rate control and variability
- PMID: 25092335
- PMCID: PMC4143073
- DOI: 10.1073/pnas.1401883111
Robust efficiency and actuator saturation explain healthy heart rate control and variability
Abstract
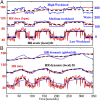
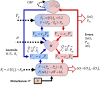
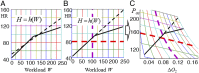
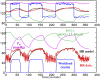
The correlation of healthy states with heart rate variability (HRV) using time series analyses is well documented. Whereas these studies note the accepted proximal role of autonomic nervous system balance in HRV patterns, the responsible deeper physiological, clinically relevant mechanisms have not been fully explained. Using mathematical tools from control theory, we combine mechanistic models of basic physiology with experimental exercise data from healthy human subjects to explain causal relationships among states of stress vs. health, HR control, and HRV, and more importantly, the physiologic requirements and constraints underlying these relationships. Nonlinear dynamics play an important explanatory role--most fundamentally in the actuator saturations arising from unavoidable tradeoffs in robust homeostasis and metabolic efficiency. These results are grounded in domain-specific mechanisms, tradeoffs, and constraints, but they also illustrate important, universal properties of complex systems. We show that the study of complex biological phenomena like HRV requires a framework which facilitates inclusion of diverse domain specifics (e.g., due to physiology, evolution, and measurement technology) in addition to general theories of efficiency, robustness, feedback, dynamics, and supporting mathematical tools.
Keywords: optimal control; respiratory sinus arrhythmia; system identification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chandra FA, Buzi G, Doyle JC. Glycolytic oscillations and limits on robust efficiency. Science. 2011;333(6039):187–192. - PubMed
-
- Pool R. Is it healthy to be chaotic? Science. 1989;243(4891):604–607. - PubMed
-
- Buzsáki G. Rhythms of the Brain. New York: Oxford Univ Press; 2006.
-
- Glass L. Synchronization and rhythmic processes in physiology. Nature. 2001;410(6825):277–284. - PubMed
-
- Buchman TG. The community of the self. Nature. 2002;420(6912):246–251. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

